Long-term effectiveness and tolerability of dolutegravir/lamivudine in treatment-naive people with HIV: an analysis of a multicentre cohort at 96 weeks
- PMID: 39710424
- PMCID: PMC11879157
- DOI: 10.1093/jac/dkae456
Long-term effectiveness and tolerability of dolutegravir/lamivudine in treatment-naive people with HIV: an analysis of a multicentre cohort at 96 weeks
Abstract
Objectives: To evaluate the long-term effectiveness, persistence and tolerability of dolutegravir (DTG)/lamivudine (3TC), compared with the most frequently prescribed first-line treatment regimens, among antiretroviral-naive people with HIV from CoRIS, a multicentre cohort in Spain, in 2018-23.
Methods: We used multivariable regression models to compare viral suppression (VS) (HIV RNA viral load <50 copies/mL), change in CD4 cell counts, persistence and treatment discontinuations due to adverse events (AEs), at 96 (±24) weeks after treatment initiation.
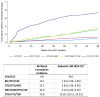
Results: Of 2359 participants, DTG/3TC was prescribed in 472 (20.0%), bictegravir/tenofovir alafenamide (TAF)/emtricitabine (FTC) in 1134 (48.1%), DTG + tenofovir disoproxil fumarate/FTC in 300 (12.7%), DTG/abacavir/3TC in 273 (11.6%) and darunavir/cobicistat/TAF/FTC in 180 (7.6%). At 96 weeks from treatment initiation, 94.0% of participants initiating with DTG/3TC achieved VS, and the mean increase in CD4 cell counts was 295.5 cells/μL (95% CI: 269.9-321.1). During the first 96 weeks after DTG/3TC initiation, 9.8% and 1.3% discontinued their initial regimen, overall and due to AEs, respectively. In multivariable analyses, we did not find significant differences in VS or increase in CD4 cell counts among participants initiating with DTG/3TC compared with other regimens. Initiating ART with a regimen other than DTG/3TC was associated with a higher risk of treatment discontinuation, overall and due to AEs.
Conclusions: Among treatment-naive people with HIV from this large multicentre cohort, DTG/3TC had similar effectiveness and better persistence and tolerability than those of the most frequently prescribed first-line regimens at 96 weeks.
© The Author(s) 2024. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures
References
-
- Cahn P, Madero JS, Arribas JR et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143–55. 10.1016/S0140-6736(18)32462-0. - DOI - PubMed
-
- European AIDS Clinical Society . EACS guidelines version 12.0. October 2023. 2023. https://www.eacsociety.org/media/guidelines-12.0.pdf.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Sciences . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. 2024. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/ad....
-
- División Panel de expertos de GeSIDA y División de control de VIH I, Hepatitis virales y, Tuberculosis. Plan Nacional sobre el SIDA . Documento de consenso de GeSIDA/Plan Nacional sobre el Sida respecto al tratamiento antirretroviral en adultos infectados por el virus de la inmunodeficiencia humana (Actualización enero 2022). 2022. https://gesida-seimc.org/wp-content/uploads/2023/01/GuiaGeSIDAPlanNacion....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

