Single-pulse transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not directly affect muscle sympathetic nerve activity in humans
- PMID: 39710610
- PMCID: PMC11663512
- DOI: 10.1093/cercor/bhae484
Single-pulse transcranial magnetic stimulation of the dorsolateral prefrontal cortex does not directly affect muscle sympathetic nerve activity in humans
Abstract
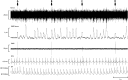
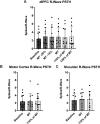
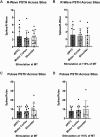
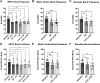
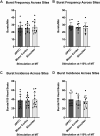
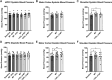
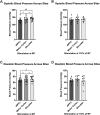
Transcranial magnetic stimulation (TMS) is applied both in research settings and clinically, notably in treating depression through the dorsolateral prefrontal cortex (dlPFC). We have recently shown that transcranial alternating current stimulation of the dlPFC partially entrains muscle sympathetic nerve activity (MSNA) to the stimulus. We, therefore, aimed to further explore the sympathetic properties of the dlPFC, hypothesizing that single-pulse TMS could generate de novo MSNA bursts. Microneurography was performed on the right common peroneal nerve in 12 participants. TMS pulses were then delivered to the ipsilateral dlPFC at resting motor threshold (MT) of the first dorsal interosseous muscle and at powers 20 below, 10 below, 10% above, and 20% above MT. The MT and 10% above MT intensities were also used to stimulate the right motor cortex and shoulder. Comparisons between stimulus intensities at the same site and between sites at the same intensities revealed no differences in MSNA burst frequency, burst incidence, or single MSNA spikes. Most stimulus trains, however, showed reduced burst frequency and incidence from baseline, regardless of site. This suggests that the TMS itself was evoking arousal-based sympathoinhibition, independent of dlPFC influences. It seems the dlPFC is capable of modulating MSNA but cannot directly generate bursts.
Keywords: dorsolateral prefrontal cortex; motor cortex; muscle sympathetic nerve activity; transcranial magnetic stimulation.
© The Author(s) 2024. Published by Oxford University Press.
Figures
Similar articles
-
Non-additive effects of electrical stimulation of the dorsolateral prefrontal cortex and the vestibular system on muscle sympathetic nerve activity in humans.Exp Brain Res. 2024 Jul;242(7):1773-1786. doi: 10.1007/s00221-024-06852-5. Epub 2024 Jun 1. Exp Brain Res. 2024. PMID: 38822824 Free PMC article.
-
The effects of electrical stimulation of ventromedial prefrontal cortex on skin sympathetic nerve activity.Cereb Cortex. 2024 Jun 4;34(6):bhae235. doi: 10.1093/cercor/bhae235. Cereb Cortex. 2024. PMID: 38839074
-
Modulation of cortical responses by transcranial direct current stimulation of dorsolateral prefrontal cortex: A resting-state EEG and TMS-EEG study.Brain Stimul. 2018 Sep-Oct;11(5):1024-1032. doi: 10.1016/j.brs.2018.06.004. Epub 2018 Jun 18. Brain Stimul. 2018. PMID: 29921529 Clinical Trial.
-
Electrical stimulation of the ventromedial prefrontal cortex modulates muscle sympathetic nerve activity and blood pressure.Cereb Cortex. 2024 Jan 14;34(1):bhad422. doi: 10.1093/cercor/bhad422. Cereb Cortex. 2024. PMID: 37950875
-
The role of the dorsolateral prefrontal cortex in control of skin sympathetic nerve activity in humans.Cereb Cortex. 2023 Jun 20;33(13):8265-8272. doi: 10.1093/cercor/bhad112. Cereb Cortex. 2023. PMID: 37143172 Free PMC article.
References
-
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985:325:1106–1107. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

