3D deuterium metabolic imaging (DMI) of the human liver at 7 T using low-rank and subspace model-based reconstruction
- PMID: 39710859
- PMCID: PMC11893041
- DOI: 10.1002/mrm.30395
3D deuterium metabolic imaging (DMI) of the human liver at 7 T using low-rank and subspace model-based reconstruction
Abstract
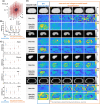
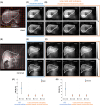
Purpose: To implement a low-rank and subspace model-based reconstruction for 3D deuterium metabolic imaging (DMI) and compare its performance against Fourier transform-based (FFT) reconstruction in terms of spectral fitting reliability.
Methods: Both reconstruction methods were applied on simulated and experimental DMI data. Numerical simulations were performed to evaluate the effect of increasing acceleration factors. The impact on spectral fitting results, SNR, and the overall normalized root mean square error (NRMSE) compared to ground-truth data were calculated. A comparative analysis was performed on DMI data acquired from the human liver, including both natural abundance and post-deuterated glucose intake data at 7 T.
Results: Simulation showed the Cramer-Rao lower bound [%] of water, glucose, sum of glutamate and glutamine (Glx), and lipid signals for the low-rank and subspace model-based reconstruction at R = 1.0 was 12.4, 14.7, 17.3, and 11.0 times lower than FFT. At R = 1.1, NRMSE was 1.4%, 1.3%, 0.8%, and 4.2% lower for the water, glucose, Glx, and lipid, respectively, compared to FFT. However, the NRMSE of the Glx and lipid increased by 0.4% and 3.2% at R = 1.3. For the in vivo DMI experiment, SNR was 2.5-3.0 times higher compared to FFT. The fitted amplitude of water and glucose peaks showed Cramer-Rao lower bound [%] values that were approximately 2.3 times lower than FFT.
Conclusion: Simulations and in vivo experiments on the human liver demonstrate that low-rank and subspace model-based reconstruction with undersampled data mitigates noise and enhances spectral fitting quality.
Keywords: 2H; 7 T; DMI; SPICE; deuterium; deuterium MRSI; liver; low rank; subspace model.
© 2024 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures



Similar articles
-
Whole-brain deuterium metabolic imaging via concentric ring trajectory readout enables assessment of regional variations in neuronal glucose metabolism.Hum Brain Mapp. 2024 Apr 15;45(6):e26686. doi: 10.1002/hbm.26686. Hum Brain Mapp. 2024. PMID: 38647048 Free PMC article.
-
Deuterium metabolic imaging of the human brain in vivo at 7 T.Magn Reson Med. 2023 Jan;89(1):29-39. doi: 10.1002/mrm.29439. Epub 2022 Sep 5. Magn Reson Med. 2023. PMID: 36063499 Free PMC article.
-
Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo.Sci Adv. 2018 Aug 22;4(8):eaat7314. doi: 10.1126/sciadv.aat7314. eCollection 2018 Aug. Sci Adv. 2018. PMID: 30140744 Free PMC article.
-
High-resolution (1) H-MRSI of the brain using SPICE: Data acquisition and image reconstruction.Magn Reson Med. 2016 Oct;76(4):1059-70. doi: 10.1002/mrm.26019. Epub 2015 Oct 28. Magn Reson Med. 2016. PMID: 26509928 Free PMC article.
-
Metabolic imaging with deuterium labeled substrates.Prog Nucl Magn Reson Spectrosc. 2023 Apr-Jun;134-135:39-51. doi: 10.1016/j.pnmrs.2023.02.002. Epub 2023 Feb 10. Prog Nucl Magn Reson Spectrosc. 2023. PMID: 37321757 Review.
Cited by
-
Comparison of Low-Rank Denoising Methods for Dynamic Deuterium MRSI at 7 T.NMR Biomed. 2025 Oct;38(10):e70125. doi: 10.1002/nbm.70125. NMR Biomed. 2025. PMID: 40887895 Free PMC article.
-
Feasibility of High-Resolution Deuterium Metabolic Imaging of the Human Kidney Using Concentric Ring Trajectory Sampling at 7T.NMR Biomed. 2025 Oct;38(10):e70139. doi: 10.1002/nbm.70139. NMR Biomed. 2025. PMID: 40923708 Free PMC article.
References
-
- Warburg VO. Über den Stoffwechsel der Carcinomzelle. Naturwissenschaften. 1924;12:1131‐1137.
MeSH terms
Substances
Grants and funding
- 813120/European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement
- 20209/The information Technology for European Advancement program (ITEA4)
- 101058229/MITI (EU EIC Transition) Non-ionizing Metabolic Imaging for predicting the effect of and guiding Therapeutic Interventions
LinkOut - more resources
Full Text Sources
Medical

