Single-molecule orientation-localization microscopy: Applications and approaches
- PMID: 39710866
- PMCID: PMC11771422
- DOI: 10.1017/S0033583524000167
Single-molecule orientation-localization microscopy: Applications and approaches
Abstract
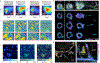
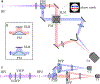
Single-molecule orientation-localization microscopy (SMOLM) builds upon super-resolved localization microscopy by imaging orientations and rotational dynamics of individual molecules in addition to their positions. This added dimensionality provides unparalleled insights into nanoscale biophysical and biochemical processes, including the organization of actin networks, movement of molecular motors, conformations of DNA strands, growth and remodeling of amyloid aggregates, and composition changes within lipid membranes. In this review, we discuss recent innovations in SMOLM and cover three key aspects: (1) biophysical insights enabled by labeling strategies that endow fluorescent probes to bind to targets with orientation specificity; (2) advanced imaging techniques that leverage the physics of light-matter interactions and estimation theory to encode orientation information with high fidelity into microscope images; and (3) computational methods that ensure accurate and precise data analysis and interpretation, even in the presence of severe shot noise. Additionally, we compare labeling approaches, imaging hardware, and publicly available analysis software to aid the community in choosing the best SMOLM implementation for their specific biophysical application. Finally, we highlight future directions for SMOLM, such as the development of probes with improved photostability and specificity, the design of “smart” adaptive hardware, and the use of advanced computational approaches to handle large, complex datasets. This review underscores the significant current and potential impact of SMOLM in deepening our understanding of molecular dynamics, paving the way for future breakthroughs in the fields of biophysics, biochemistry, and materials science.
Keywords: biophysical chemistry and spectroscopy; fluorescence; physical chemistry; single-molecule dichroism; single-molecule fluorescence anisotropy.
Figures
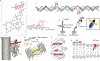


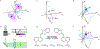


References
-
- Adams C, Gross RE, Martin LA, Walkup BR, Yao GK, Llopis JY and Tsien RY (2002) New biarsenical ligands and Tetracysteine motifs for protein labeling in vitro and in vivo: Synthesis and biological applications. Journal of the American Chemical Society 124 (21), 6063–6076. 10.1021/ja017687n - DOI - PubMed
-
- Alonso MA (2023) Geometric descriptions for the polarization of nonparaxial light: A tutorial. Advances in Optics and Photonics 15 (1), 176. 10.1364/AOP.475491 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

