Brexucabtagene autoleucel in-vivo expansion and BTKi refractoriness have a negative influence on progression-free survival in mantle cell lymphoma: Results from CART-SIE study
- PMID: 39710966
- PMCID: PMC11829141
- DOI: 10.1111/bjh.19961
Brexucabtagene autoleucel in-vivo expansion and BTKi refractoriness have a negative influence on progression-free survival in mantle cell lymphoma: Results from CART-SIE study
Abstract
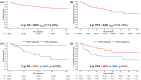
Brexucabtagene autoleucel (brexu-cel) has revolutionized the treatment of patients affected by mantle cell lymphomas. In this prospective, observational multicentre study, we evaluated 106 patients, with longitudinal brexu-cel kinetics in peripheral blood monitored in 61 of them. Clinical outcomes and toxicities are consistent with previous real-world evidence studies. Notably, beyond established poor prognostic factors-such as blastoid variant and elevated lactate dehydrogenase-Bruton tyrosine-kinase inhibitors (BTKi) refractoriness and platelet count emerged as significant predictors of survival. Specifically, the 1-year overall survival was 56% in BTKi-refractory patients compared to 92% in BTKi-relapsed patients (p = 0.0001). Our study also demonstrated that in-vivo monitoring of brexu-cel expansion is feasible and correlates with progression-free survival and toxicities. Progression-free survival at 1 year was 74% in patients categorized as strong expanders, based on brexu-cel peak concentration, versus 54% in poor expanders (p = 0.02). Furthermore, in-vivo expansion helped identify a high-risk group of non-responders, those with progressive or stable disease at the 90-day post-infusion evaluation (OR = 4.7, 95% CI = 1.1-34, p = 0.04) characterized by dismal outcomes. When integrated with other clinical factors, monitoring brexu-cel expansion could assist in recognizing patients at high risk of early relapse.
Keywords: CAR T‐cell; brexu‐cel; in vivo expansion; mantle cell lymphoma; real world.
© 2024 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
Federico Stella—nothing to disclose; Annalisa Chiappella—reports other support from Gilead Sciences, Ideogen, Roche, Secura Bio, Takeda, AbbVie, Eli Lilly and Company, Incyte, Janssen‐Cilag, and Novartis outside the submitted work; Martina Magni—nothing to disclose; Francesca Bonifazi—fees from Novartis and Kite‐Gilead; Chiara De Philippis—nothing to disclose; Maurizio Musso—advisory board Kite/Gilead; Novartis; BMS. Speakers bureau: Kite/Gilead; Novartis; BMS; Ilaria Cutini—nothing to disclose, Silva Ljevar—nothing to disclose; Anna Maria Barbui—nothing to disclose; Mirko Farina—nothing to disclose; Massimo Martino—advisory board Kite/Gilead; Novartis; BMS. Speakers bureau: Kite/Gilead; Novartis; BMS; Massimo Massaia—nothing to disclose; Giovanni Grillo—nothing to disclose; Piera Angelillo—Incyte, Barbara Botto—nothing to disclose, Francesca Patriarca—Sanofi, Menarini, Novartis, BMS; Mauro Krampera—Advisory boards and honoraria for lectures/educational events: Kite Gilead, Novartis, Janssen‐Cilag, Incyte, AbbVie, BeiGene, Menarini StemLine, Roche; Luca Arcaini—Honoraria: EUSA Pharma, Novartis. Advisory boards for Roche, Janssen‐Cilag, Verastem, Incyte, EUSA Pharma, Celgene/Bristol Myers Squibb, Kite/Gilead, ADC Therapeutics, Novartis; Maria Chiara Tisi—personal fees from Novartis, Gilead, Bristol Myers Squibb, Eli Lilly and Company, Janssen, Sobi and Incyte; Pierluigi Zinzani—Consultant: MSD, Eusapharma, Novartis; Advisory boards: ADC Therapeutics, Astrazeneca, BeiGene, BMS, Celltrion, Eusapharma, Gilead, Incyte, Janssen‐Cilag, KyowaKirin, MSD, Novartis, Roche, Sandoz, SecuraBio, Servier, Takeda; speakers bureau: Astrazeneca, Beigene, BMS, Celltrion, Eusapharma, Gilead, Incyte, Janssen‐Cilag, Kyowa‐Kirin, MSD, Novartis, Roche, Servier, Takeda; Federica Sorà—nothing to disclose; Stefania Bramanti—Speaker bureau: Bms; Gilead, Novartis, Advisory Board Novartis, Travel Accomodation Novartis, Roche, Martina Pennisi—nothing to disclose, Cristiana Carniti—nothing to disclose, Paolo Corradini—Advisory boards: AbbVie, ADC Therapeutics, Amgen, BeiGene, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda; honoraria for lectures: AbbVie, Amgen, Celgene, Gilead/Kite, Janssen, Novartis, Roche, Sanofi, Takeda.
Figures

References
-
- Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner‐Johnston N, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. https://ashpublications.org/blood/article/127/12/1559/35048/Postibrutini... - PubMed

