Structure-Based Discovery and Development of Highly Potent Dihydroorotate Dehydrogenase Inhibitors for Malaria Chemoprevention
- PMID: 39710971
- PMCID: PMC11726676
- DOI: 10.1021/acs.jmedchem.4c02394
Structure-Based Discovery and Development of Highly Potent Dihydroorotate Dehydrogenase Inhibitors for Malaria Chemoprevention
Abstract
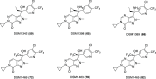
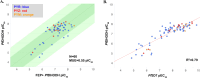
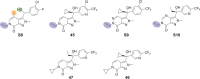
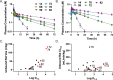
Malaria remains a serious global health challenge, yet treatment and control programs are threatened by drug resistance. Dihydroorotate dehydrogenase (DHODH) was clinically validated as a target for treatment and prevention of malaria through human studies with DSM265, but currently no drugs against this target are in clinical use. We used structure-based computational tools including free energy perturbation (FEP+) to discover highly ligand efficient, potent, and selective pyrazole-based Plasmodium DHODH inhibitors through a scaffold hop from a pyrrole-based series. Optimized pyrazole-based compounds were identified with low nM-to-pM Plasmodium falciparum cell potency and oral activity in a humanized SCID mouse malaria infection model. The lead compound DSM1465 is more potent and has improved absorption, distribution, metabolism and excretion/pharmacokinetic (ADME/PK) properties compared to DSM265 that support the potential for once-monthly chemoprevention at a low dose. This compound meets the objective of identifying compounds with potential to be used for monthly chemoprevention in Africa to support malaria elimination efforts.
Conflict of interest statement
The authors declare the following competing financial interest(s): RB, ZN, MAP, SAC, VF, CH, JP, TQ, MJP and BL are named inventors on a patent describing compounds contained in this manuscript; BL is an employee of MMV, RB and MJP are and were MMV consultants, respectively.
Figures




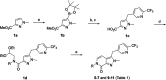
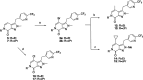
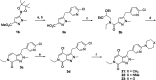


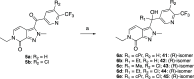
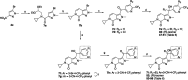
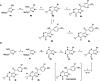


References
-
- WHO World Malaria Report 2023. (accessed Sept 14 2024). https://www.who.int/teams/global-malaria-programme/reports/world-malaria....
-
- Dondorp A. M.; Nosten F.; Yi P.; Das D.; Phyo A. P.; Tarning J.; Lwin K. M.; Ariey F.; Hanpithakpong W.; Lee S. J.; Ringwald P.; Silamut K.; Imwong M.; Chotivanich K.; Lim P.; Herdman T.; An S. S.; Yeung S.; Singhasivanon P.; Day N. P.; Lindegardh N.; Socheat D.; White N. J. Artemisinin Resistance in Plasmodium falciparum Malaria. N. Engl. J. Med. 2009, 361, 455–467. 10.1056/NEJMoa0808859. - DOI - PMC - PubMed
-
- Phyo A. P.; Ashley E. A.; Anderson T. J. C.; Bozdech Z.; Carrara V. I.; Sriprawat K.; Nair S.; White M. M.; Dziekan J.; Ling C.; Proux S.; Konghahong K.; Jeeyapant A.; Woodrow C. J.; Imwong M.; McGready R.; Lwin K. M.; Day N. P. J.; White N. J.; Nosten F. Declining Efficacy of Artemisinin Combination Therapy Against P. falciparum Malaria on the Thai-Myanmar Border (2003–2013): The Role of Parasite Genetic factors. Clin. Infect. Dis. 2016, 63, 784–791. 10.1093/cid/ciw388. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

