Trifluoroacetic Acid as a Molecular Probe for the Dense Phase in Liquid-Liquid Phase-Separating Peptide Systems
- PMID: 39710972
- PMCID: PMC11740181
- DOI: 10.1021/acs.analchem.4c03444
Trifluoroacetic Acid as a Molecular Probe for the Dense Phase in Liquid-Liquid Phase-Separating Peptide Systems
Abstract
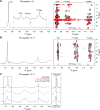
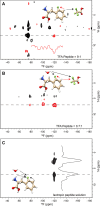
Although trifluoroacetic acid (TFA) is not typically considered a Hofmeister reagent, it has been demonstrated to modulate biocoacervation. We show that TFA can be employed to probe specific interactions in coacervating bioinspired peptide phenylalanine (Phe) 19F-labeled at a single site, altering its liquid-liquid phase separation (LLPS) behavior. Solid-state nuclear magnetic resonance (NMR) spectroscopy revealed two dynamically distinct binding modes of TFA with Phe, resulting in a structured, dipolar-ordered complex and a more dynamic complex, highlighting the proximity between TFA and Phe. Quantum chemistry modeling of 19F chemical shift differences indicates that the structured complex is formed by the intercalation of one TFA molecule between two stacked Phe aromatic rings, possibly contributing to the stabilization of the condensed dense phase. Thus, we propose that TFA can be used as a convenient molecular probe in 19F NMR-based studies of the structure and dynamics of the dense phase in LLPS peptide systems.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Harrington M. J.; Mezzenga R.; Miserez A. Fluid protein condensates for bio-inspired applications. Nat. Rev. Bioeng. 2024, 2 (3), 260–278. 10.1038/s44222-023-00133-6. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

