Metabolic Stress Expands Polyfunctional, Proinflammatory Th17 Cells in Patients With Psoriatic Arthritis for Whom There is Interleukin-23-Independent Interleukin-17 Production
- PMID: 39711043
- PMCID: PMC12209755
- DOI: 10.1002/art.43095
Metabolic Stress Expands Polyfunctional, Proinflammatory Th17 Cells in Patients With Psoriatic Arthritis for Whom There is Interleukin-23-Independent Interleukin-17 Production
Abstract
Objective: Genetic associations and blockade of the interleukin (IL)-23/IL-17 axis with monoclonal antibodies support a role for this pathway in patients with psoriatic arthritis (PsA). This study examines the requirement of IL-23 for IL-17 production and the role of the metabolic microenvironment in the expansion of Th17-derived cells in patients with PsA.
Methods: Th17 cell frequencies in synovial fluid or peripheral blood from patients with PsA were evaluated by flow cytometry using chemokine receptor 6, CD161, and T-bet as phenotypic markers, and the cytokines interferon γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-17 were assessed by flow cytometry and enzyme-linked immunosorbent assay. The impact of IL-23 and metabolic stress on T cell differentiation was investigated.
Results: Polyfunctional positive IL-17 (IL-17pos) CD4 (P < 0.0001) and CD8 (P < 0.0001), and GM-CSFpos Th17-derived cells (P < 0.0001) were increased in the inflamed joints of patients with PsA, with a proportional decrease in the peripheral blood of patients. We demonstrate IL-23-independent IL-17 release by CD4 T cells in patients with PsA, in which the absence of IL-23 during Th17 differentiation reduced IL-17 by mean ± SEM 31% ± 5.8%. Exogenous IL-23 increased IL-17, negatively regulated GM-CSF, and cooperated with transforming growth factor β to augment IL-17. Polyfunctional Th17 and Th17-derived cells, but not Th1 cells, were expanded by metabolic stress in patients with PsA.
Conclusion: We confirmed the abundance of polyfunctional type 17 CD4 and CD8 cells in the inflamed joints of patients with PsA. We demonstrate IL-23-independent expansion of Th17 cells, for which IL-23 negatively regulates GM-CSF. This may account for therapeutic differences in IL-17 and IL-23 inhibition in patients with PsA or other spondyloarthritides. Polyfunctional IL-17pos Th17 and Th17-derived but not Th1 cells were expanded by metabolic stress, and metabolic stress may itself represent a unique therapeutic target.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

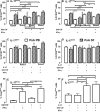

 ) with exogenous IL‐23, IL‐12, or IL‐2. Samples from patients with PsA were also compared with PBMCs from HDs within treatment groups. Data are represented as paired samples, with data analyzed by paired or unpaired Student's t‐test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ELISA, enzyme‐linked immunosorbent assay; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; HD, healthy donor; IFN, interferon; IL, interleukin; PB, peripheral blood; PBMC, PB mononuclear cell; PsA, psoriatic arthritis.
) with exogenous IL‐23, IL‐12, or IL‐2. Samples from patients with PsA were also compared with PBMCs from HDs within treatment groups. Data are represented as paired samples, with data analyzed by paired or unpaired Student's t‐test. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. ELISA, enzyme‐linked immunosorbent assay; GM‐CSF, granulocyte–macrophage colony‐stimulating factor; HD, healthy donor; IFN, interferon; IL, interleukin; PB, peripheral blood; PBMC, PB mononuclear cell; PsA, psoriatic arthritis.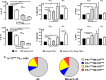

References
-
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol 2017;31(2):205–212. - PubMed
-
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol 2016;68(4):915–923. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

