Transcranial Direct Current Stimulation Over Bilateral Temporal Lobes Modulates Hippocampal-Occipital Functional Connectivity and Visual Short-Term Memory Precision
- PMID: 39711102
- PMCID: PMC11664227
- DOI: 10.1002/hipo.23678
Transcranial Direct Current Stimulation Over Bilateral Temporal Lobes Modulates Hippocampal-Occipital Functional Connectivity and Visual Short-Term Memory Precision
Abstract
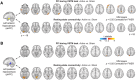
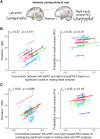
Although the medial temporal lobe (MTL) is traditionally considered a region dedicated to long-term memory, recent neuroimaging and intracranial recording evidence suggests that the MTL also contributes to certain aspects of visual short-term memory (VSTM), such as the quality or precision of retained VSTM content. This study aims to further investigate the MTL's role in VSTM precision through the application of transcranial direct current stimulation (tDCS) and functional magnetic resonance imaging (fMRI). Participants underwent 1.5 mA offline tDCS over bilateral temporal lobes using left cathodal and right anodal electrodes, administered for either 20 min (active) or 0.5 min within a 20-min window (sham), in a counterbalanced design. As the electrical current passes through midbrain structures with this bilateral stimulation montage, prior behavioral and modeling evidence suggests that this tDCS protocol can modulate MTL functions. To confirm this and examine its impacts on VSTM, participants completed a VSTM color recall task immediately following tDCS, while undergoing a 20-min fMRI scan and a subsequent 7.5-min resting-state scan, during which they focused on a fixation cross. Behavioral results indicated that this tDCS protocol decreased VSTM precision without significantly affecting overall recall success. Furthermore, psychophysiological interaction analysis revealed that tDCS over the temporal lobe modulated hippocampal-occipital functional connectivity during the VSTM task, despite no main effect on fMRI BOLD activity. Notably, this modulation was also observed during resting-state fMRI 15-20 min post-tDCS, with the magnitude of the effect correlating with participants' behavioral changes in VSTM precision across active and control conditions. Combined, these findings suggest that tDCS over the temporal lobe can modulate the intrinsic functional connectivity between the MTL and visual sensory areas, thereby affecting VSTM precision.
Keywords: fMRI; medial temporal lobe; pattern separation; precision; tDCS; visual short‐term memory.
© 2024 The Author(s). Hippocampus published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Electrical Stimulation Over Human Posterior Parietal Cortex Selectively Enhances the Capacity of Visual Short-Term Memory.J Neurosci. 2019 Jan 16;39(3):528-536. doi: 10.1523/JNEUROSCI.1959-18.2018. Epub 2018 Nov 20. J Neurosci. 2019. PMID: 30459222 Free PMC article.
-
Influence of noninvasive brain stimulation on connectivity and local activation: a combined tDCS and fMRI study.Eur Arch Psychiatry Clin Neurosci. 2024 Jun;274(4):827-835. doi: 10.1007/s00406-023-01666-y. Epub 2023 Aug 19. Eur Arch Psychiatry Clin Neurosci. 2024. PMID: 37597023 Free PMC article.
-
Visual Short-Term Memory Activity in Parietal Lobe Reflects Cognitive Processes beyond Attentional Selection.J Neurosci. 2018 Feb 7;38(6):1511-1519. doi: 10.1523/JNEUROSCI.1716-17.2017. Epub 2018 Jan 8. J Neurosci. 2018. PMID: 29311140 Free PMC article.
-
Transcranial electrical stimulation of the occipital cortex during visual perception modifies the magnitude of BOLD activity: A combined tES-fMRI approach.Neuroimage. 2016 Oct 15;140:110-7. doi: 10.1016/j.neuroimage.2015.11.034. Epub 2015 Nov 23. Neuroimage. 2016. PMID: 26608246
-
Prefrontal and Medial Temporal Lobe Cortical Contributions to Visual Short-Term Memory.J Cogn Neurosci. 2022 Dec 1;35(1):27-43. doi: 10.1162/jocn_a_01937. J Cogn Neurosci. 2022. PMID: 36306260 Review.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

