A Chemically Robust Microporous Zn-MOF for C2H2 Separation from CO2 and Industrially Relevant Four Component Gas Mixtures
- PMID: 39711264
- PMCID: PMC11855262
- DOI: 10.1002/smll.202411456
A Chemically Robust Microporous Zn-MOF for C2H2 Separation from CO2 and Industrially Relevant Four Component Gas Mixtures
Abstract
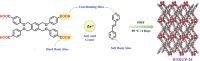
The separation and purification of acetylene from the light hydrocarbon gas mixtures is considered as one of the most industrially challenging task for the production of fine chemicals. Though metal-organic frameworks (MOFs) are promising candidates for such separation and offer a cost and energy-efficient pathway, achieving the trade-off between sorption capacity and separation selectivity along with framework robustness is a daunting task and demands effective design. Herein, a new 3D chemically stable MOF, IITKGP-24 (stable over a wide range of aqueous pH solution, pH = 2-12) is developed, displaying excellent separation selectivity of 13.9 for C2H2/CO2 (50:50) even at ambient conditions and maintained a trade-off between sorption capacity and separation selectivity. Most importantly, the breakthrough performance analysis under the industrially relevant gas mixture composition revealed that the developed framework possesses excellent separation of acetylene from not only C2H2/CO2 (50:50) gas mixtures but also from the quaternary C2H2/C2H4/C2H6/CO2 (25:25:25:25) feed gas streams. Separation of C2H2 from such a four component gas mixture by MOFs is unexplored. The exceptional framework robustness, high C2H2/CO2 uptake ratio, low heat of adsorption, and excellent recyclability with easy regenerability made the developed framework promising candidate toward this challenging separation.
Keywords: acetylene purification; multi‐component separation; pH‐stable MOF; physisorbent materials; surface functionalization.
© 2024 The Author(s). Small published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sholl D. S., Lively R. P., Nature 2016, 532, 435. - PubMed
-
- Wang X., Liu H., Li Y., Yang X., Gao F., Wang X., Kang Z., Fan W., Sun D., Coord. Chem. Rev. 2023, 482, 215093.
-
- Sahoo R., Das M. C., Coord. Chem. Rev. 2021, 442, 213998.
-
- Sahoo R., Chand S., Mondal M., Pal A., Pal S. C., Rana M. K., Das M. C., Chem. ‐ Eur. J. 2020, 26, 12624. - PubMed
-
- Sahoo R., Mondal S., Mukherjee D., Das M. C., Adv. Funct. Mater. 2022, 32, 2207197.
LinkOut - more resources
Full Text Sources

