Zebrafish Dark-Dependent Behavior Requires Phototransduction by the Pineal Gland
- PMID: 39711421
- PMCID: PMC11664460
- DOI: 10.1111/jpi.70021
Zebrafish Dark-Dependent Behavior Requires Phototransduction by the Pineal Gland
Abstract
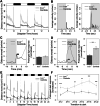
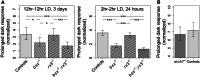
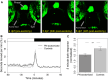
Located dorsally underneath a thin translucent skull in many teleosts, the pineal gland is a photoreceptive organ known as a key element of the circadian clock system. Nevertheless, the presence of additional routes of photoreception presents a challenge in determining its specific roles in regulating photic-related behavior. Here, we show the importance of the pineal gland in mediating a prolonged motor response of zebrafish larvae to sudden darkness, both as a photodetector and as a circadian pacemaker. This was evident by a reduced motor response of Bsx-deficient larvae, lacking a pineal gland, to sudden darkness. Moreover, the typical daily rhythm of the intensity of this response was lost in the pineal-less larvae. In contrast, motor response to a sudden increase in illumination was unaffected. Furthermore, we show that the pineal-mediated behavioral response to darkness requires two elements: the photoreceptor cells and the projecting neurons. Dark response was impaired in larvae whose pineal photoreceptor cells were genetically ablated and in larvae whose pineal projecting neurons had undergone laser-axotomy. This study thus establishes the pineal gland as a mediator of dark-dependent behavior and reveals underlying cellular components involved in transducing information about darkness to the brain.
Keywords: VMR; brain‐specific homeobox (Bsx); photoreceptors; pineal; projecting neurons; zebrafish.
© 2024 The Author(s). Journal of Pineal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ekstrzm P. and Meissl H., “The Pineal Organ of Teleost Fishes,” Reviews in Fish Biology and Fisheries 7 (1997): 199–284.
-
- Bertolucci C. and Foà A., “Extraocular Photoreception and Circadian Entrainment in Nonmammalian Vertebrates,” Chronobiology International 21, no. 4–5 (2004): 501–519. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

