Melatonin Inhibits Endometriosis Growth via Specific Binding and Inhibition of EGFR Phosphorylation
- PMID: 39711424
- PMCID: PMC11664468
- DOI: 10.1111/jpi.70022
Melatonin Inhibits Endometriosis Growth via Specific Binding and Inhibition of EGFR Phosphorylation
Abstract
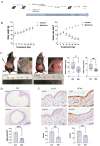
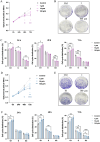
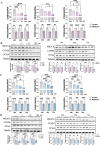
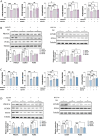
As a chronic gynecological disease, endometriosis is defined as the implantation of endometrial glands as well as stroma outside the uterine cavity. Proliferation is a major pathophysiology in endometriosis. Previous studies demonstrated a hormone named melatonin, which is mainly produced by the pineal gland, exerts a therapeutic impact on endometriosis. Despite that, the direct binding targets and underlying molecular mechanism have remained unknown. Our study revealed that melatonin treatment might be effective in inhibiting the growth of lesions in endometriotic mouse model as well as in human endometriotic cell lines. Additionally, the drug-disease protein-protein interaction (PPI) network was built, and epidermal growth factor receptor (EGFR) was identified as a new binding target of melatonin treatment in endometriosis. Computational simulation together with BioLayer interferometry was further applied to confirm the binding affinity. Our result also showed melatonin inhibited the phosphorylation level of EGFR not only in endometriotic cell lines but also in mouse models. Furthermore, melatonin inhibited the phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-protein kinase B (Akt) pathway and arrested the cell cycle via inhibiting CyclinD1 (CCND1). In vitro and in vivo knockdown/restore assays further demonstrated the involvement of the binding target and signaling pathway that we found. Thus, melatonin can be applied as a novel therapy for the management of endometriosis.
Keywords: CCND1; EGFR; PI3K/AKT; cell cycle; endometriosis; melatonin; proliferation.
© 2024 The Author(s). Journal of Pineal Research published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Melatonin inhibits endometriosis development by disrupting mitochondrial function and regulating tiRNAs.J Pineal Res. 2023 Jan;74(1):e12842. doi: 10.1111/jpi.12842. Epub 2022 Nov 27. J Pineal Res. 2023. PMID: 36401340
-
β-elemene, a sesquiterpene constituent from Curcuma phaeocaulis inhibits the development of endometriosis by inducing ferroptosis via the MAPK and STAT3 signaling pathways.J Ethnopharmacol. 2025 Feb 11;341:119344. doi: 10.1016/j.jep.2025.119344. Epub 2025 Jan 10. J Ethnopharmacol. 2025. PMID: 39800242
-
Distinct molecular targets of ProEGCG from EGCG and superior inhibition of angiogenesis signaling pathways for treatment of endometriosis.J Pharm Anal. 2024 Jan;14(1):100-114. doi: 10.1016/j.jpha.2023.09.005. Epub 2023 Sep 11. J Pharm Anal. 2024. PMID: 38352946 Free PMC article.
-
Melatonin in Endometriosis: Mechanistic Understanding and Clinical Insight.Nutrients. 2022 Oct 1;14(19):4087. doi: 10.3390/nu14194087. Nutrients. 2022. PMID: 36235740 Free PMC article. Review.
-
Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics.Hum Reprod Update. 2016 Apr;22(3):382-403. doi: 10.1093/humupd/dmv060. Epub 2016 Jan 5. Hum Reprod Update. 2016. PMID: 26740585 Review.
References
-
- Sampson J. A., “Peritoneal Endometriosis Due to the Menstrual Dissemination of Endometrial Tissue Into the Peritoneal Cavity,” American Journal of Obstetrics and Gynecology 14, no. 4 (1927): 422–469, 10.1016/s0002-9378(15)30003-x. - DOI
-
- Nap A. W., “Theories on the Pathogenesis of Endometriosis,” in Endometriosis: Science and Practice, 1st ed. (2012): 42–53, 10.1002/9781444398519.ch5. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

