Liposomal Bupivacaine Use in Third Molar Impaction Surgery: INNOVATE Study
- PMID: 39711449
- PMCID: PMC11614472
- DOI: 10.2344/333161
Liposomal Bupivacaine Use in Third Molar Impaction Surgery: INNOVATE Study
Abstract
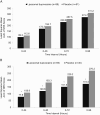
The analgesic efficacy and safety of liposomal bupivacaine (LB) in third molar extraction was evaluated in this phase 3, double-blind, placebo-controlled study of subjects undergoing bilateral third molar extraction. Subjects were randomized 2: 1 to infiltration with LB (133 mg/10 mL) or placebo, and received opioid rescue medication as needed. Primary efficacy measure was cumulative area under the curve (AUC) of numeric rating scale (NRS) pain severity scores through 48 hours (AUC of NRS0-48) postsurgery. Other measures included AUC of NRS0-24, AUC of NRS0-72, and AUC of NRS0-96, and incidence of adverse events. There were 150 subjects in the primary efficacy population (n = 99 LB, n = 51 placebo) and 89 in the per-protocol population (n = 59 LB, n = 30 placebo). Least-squares mean for AUC of NRS0-48 was 172.3 LB versus 194.7 placebo (P = .227) in the primary efficacy population and 120.8 LB versus 183.3 placebo (P = .023) in the per-protocol population. At all time points, between-group differences in AUC of NRS scores were significant in the per-protocol population (LB lower than placebo, P < .05) but not in the primary efficacy population. The adverse event profile was similar between groups. LB produced significantly lower cumulative pain scores versus placebo at all time points in the per-protocol analysis but not in the primary efficacy analysis because of protocol violations. This study indicates significant improvement in pain scores in the third molar model, but because of extensive protocol violations additional studies are warranted to demonstrate effectiveness.
Keywords: Bupivacaine; Impacted tooth; Nonnarcotic analgesics; Oral surgery; Postoperative pain; Third molar; Tooth extraction.
Figures



References
-
- Snyder M, Shugars DA, White RP, Phillips C Pain medication as an indicator of interference with lifestyle and oral function during recovery after third molar surgery. J Oral Maxillofac Surg. 2005;63:1130–1137. - PubMed
-
- Bienstock DA, Dodson TB, Perrott DH, Chuang SK Prognostic factors affecting the duration of disability after third molar removal. J Oral Maxillofac Surg. 2011;69:1272–1277. - PubMed
-
- American Society of Anesthesiologists Task Force on Acute Pain Management . Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

