Addressing Disparities Using Continuous Glucose Monitors and Remote Patient Monitoring for Youth With Type 1 Diabetes
- PMID: 39711469
- PMCID: PMC11664559
- DOI: 10.1177/19322968241305612
Addressing Disparities Using Continuous Glucose Monitors and Remote Patient Monitoring for Youth With Type 1 Diabetes
Abstract
Background: Youth with type 1 diabetes (T1D) and public insurance have lower diabetes technology use. This pilot study assessed the feasibility of a program to support continuous glucose monitor (CGM) use with remote patient monitoring (RPM) to improve glycemia for youth with established T1D and public insurance.
Methods: From August 2020 to June 2023, we provided CGM with RPM support via patient portal messaging for youth with established T1D on public insurance with challenges obtaining consistent CGM supplies. We prospectively collected hemoglobin A1c (HbA1c), standard CGM metrics, and diabetes technology use over 12 months.
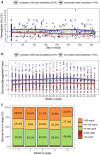
Results: The cohort included 91 youths with median age at enrollment 14.7 years, duration of diabetes 4.4 years, 33% non-English speakers, and 44% Hispanic. Continuous glucose monitor data were consistently available (≥70%) in 23% of the participants. For the 64% of participants with paired HbA1c values at enrollment and study end, the median HbA1c decreased from 9.8% to 9.0% (P < .001). Insulin pump users increased from 31 to 48 and automated insulin delivery users increased from 11 to 38.
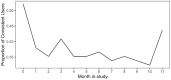
Conclusions: We established a program to support CGM use in youth with T1D and barriers to consistent CGM supplies, offering lessons for other clinics to address disparities with team-based, algorithm-enabled, remote T1D care. This real-world pilot and feasibility study noted challenges with low levels of protocol adherence and obtaining complete data in this cohort. Future iterations of the program should explore RPM communication methods that better align with this population's preferences to increase participant engagement.
Keywords: continuous glucose monitors; health disparity; pediatric diabetes; remote patient monitoring; type 1 diabetes.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Maahs has had research support from the NIH his institution has had research support from Dexcom. Dr Maahs has consulted for Abbott, the Helmsley Charitable Trust, Lifescan, Sanofi, Medtronic, Provention Bio, Kriya, Biospex, and Bayer. Dr Prahalad has consulted for Sanofi. The remaining authors do not report any relevant disclosures.
Figures

References
-
- Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171-e1179. - PubMed
-
- Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501-512. - PubMed
-
- Sherr JL, Schoelwer M, Santos TJD, et al.. ISPAD clinical practice consensus guidelines 2022: diabetes technologies: insulin delivery. Pediatr Diabetes. 2022;23(8):1406-1431. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

