Beyond BRAFV600E: Investigating the Clinical and Genetic Spectrum of Langerhans Cell Histiocytosis in Children
- PMID: 39711503
- PMCID: PMC11664316
- DOI: 10.1002/cam4.70532
Beyond BRAFV600E: Investigating the Clinical and Genetic Spectrum of Langerhans Cell Histiocytosis in Children
Abstract
Background: Langerhans cell histiocytosis (LCH) is the most prevalent histiocytic disorder in pediatric populations, with a highly heterogeneous clinical presentation. Currently, the correlation between clinical phenotypes and molecular alterations in childhood LCH, besides the BRAFV600E mutation, has not been sufficiently studied.
Methods: This study presented data on 33 pediatric LCH patients treated at our center who exhibited various molecular alterations other than the BRAFV600E mutation. Additionally, we comprehensively reviewed pediatric LCH cases with non-BRAFV600E molecular alterations reported from January 2010 to August 2024.
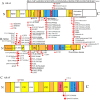
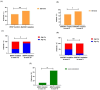
Results: A total of 309 pediatric LCH patients with molecular alterations beyond BRAFV600E were enrolled in the study, among whom 33 were from our center. In these LCH cases, 49 kinds of MAP2K1 mutations, 31 kinds of BRAF mutations, and 4 kinds of ARAF mutations were found. At our center, two patients with multisystem LCH with risk organ involvement, both with BRAFN486_P490del mutation, showed poor response to induction chemotherapy for 6 weeks. Among the 303 LCH patients with MAP2K1 or other BRAF alterations, patients with the MAP2K1 mutation had a higher prevalence of single-system bone involvement (SS-bone) than patients carrying the BRAF mutation (p = 0.0072). Within the MAP2K1 group, exon 3 mutations exhibited a stronger association with SS-bone than exon 2 mutations (p = 0.042). Additionally, patients with the BRAF exon 15 mutation and MAP2K1 exon 2 mutation had higher rates of LCH onset before age 3 compared with patients carrying the BRAF exon 12 mutation and MAP2K1 exon 3 mutation (p = 0.037; p = 0.0015). Patients carrying the BRAF mutation in exon 15 had higher rates of liver involvement compared with patients carrying the exon 12 (p = 0.042).
Conclusions: Pediatric LCH patients often carry recurrent somatic MAP2K1 and BRAF mutations, which are associated with clinical manifestations.
Keywords: BRAF; MA2PK1; Langerhans cell histiocytosis; children; clinical features.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Rodriguez‐Galindo C. and Allen C. E., “Langerhans Cell Histiocytosis,” Blood 135, no. 16 (2020): 1319–1331. - PubMed
-
- Abla O., Rollins B., and Ladisch S., “Langerhans Cell Histiocytosis: Progress and Controversies,” British Journal of Haematology 187, no. 5 (2019): 559–562. - PubMed
-
- Gadner H., Minkov M., Grois N., et al., “Therapy Prolongation Improves Outcome in Multisystem Langerhans Cell Histiocytosis,” Blood 121, no. 25 (2013): 5006–5014. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

