This is a preprint.
Desaturase-dependent secretory functions of hepatocyte-like cells control systemic lipid metabolism during starvation in Drosophila
- PMID: 39711529
- PMCID: PMC11661362
- DOI: 10.21203/rs.3.rs-5566817/v1
Desaturase-dependent secretory functions of hepatocyte-like cells control systemic lipid metabolism during starvation in Drosophila
Abstract
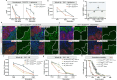
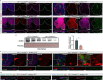
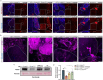
Similar to the mammalian hepatocytes, Drosophila oenocytes accumulate fat during fasting, but it is unclear how they communicate with the fat body, the major lipid source. Using a modified protocol for prolonged starvation, we show that knockdown (KD) of the sole delta 9 desaturase, Desat1 (SCD in mammals), specifically in oenocytes leads to more saturated lipids in the hemolymph and reduced triacylglycerol (TAG) storage in the fat body. Additionally, oenocytes with Desat1 KD exhibited an accumulation of lipoproteins and actin filaments at the cortex, which decreased lipoproteins in the hemolymph. We further show that ImpL2 (IGFBP7 in mammals) is secreted from oenocytes during starvation in a Desat1-dependent manner. Flies with oenocyte-specific KD and overexpression of ImpL2 exhibited higher and lower sensitivity to starvation as well as lower and higher levels of TAG, respectively. Intriguingly, the depolymerization of cortical actin in the oenocytes decreased lipoprotein sequestration and alleviated the secretion defect of Impl2 in Desat1 KD cells, leading to rescued TAG levels and starvation sensitivity. Overall, this study highlights the central role of oenocytes in systemic lipid metabolism in Drosophila as well as the importance of Desat1 in maintaining the proper functioning of oenocytes during periods of starvation.
Conflict of interest statement
Additional Declarations: There is NO Competing Interest.
Figures





Similar articles
-
Specialized hepatocyte-like cells regulate Drosophila lipid metabolism.Nature. 2007 Jan 18;445(7125):275-80. doi: 10.1038/nature05382. Epub 2006 Nov 29. Nature. 2007. PMID: 17136098
-
Roles of Insect Oenocytes in Physiology and Their Relevance to Human Metabolic Diseases.Front Insect Sci. 2022 Mar 17;2:859847. doi: 10.3389/finsc.2022.859847. eCollection 2022. Front Insect Sci. 2022. PMID: 38468774 Free PMC article. Review.
-
dMyc expression in the fat body affects DILP2 release and increases the expression of the fat desaturase Desat1 resulting in organismal growth.Dev Biol. 2013 Jul 1;379(1):64-75. doi: 10.1016/j.ydbio.2013.04.008. Epub 2013 Apr 19. Dev Biol. 2013. PMID: 23608455 Free PMC article.
-
RiboTag translatomic profiling of Drosophila oenocytes under aging and induced oxidative stress.BMC Genomics. 2019 Jan 16;20(1):50. doi: 10.1186/s12864-018-5404-4. BMC Genomics. 2019. PMID: 30651069 Free PMC article.
-
The Drosophila model to interrogate triacylglycerol biology.Biochim Biophys Acta Mol Cell Biol Lipids. 2021 Jun;1866(6):158924. doi: 10.1016/j.bbalip.2021.158924. Epub 2021 Mar 11. Biochim Biophys Acta Mol Cell Biol Lipids. 2021. PMID: 33716135 Review.
References
-
- Gutierrez E., Wiggins D., Fielding B. & Gould A. P. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445, 275–80 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

