This is a preprint.
Processed Dietary Fiber Partially Hydrolyzed Guar Gum Increases Susceptibility to Colitis and Colon Tumorigenesis in Mice
- PMID: 39711544
- PMCID: PMC11661293
- DOI: 10.21203/rs.3.rs-5522559/v1
Processed Dietary Fiber Partially Hydrolyzed Guar Gum Increases Susceptibility to Colitis and Colon Tumorigenesis in Mice
Update in
-
Processed dietary fiber partially hydrolyzed guar gum increases susceptibility to colitis and colon tumorigenesis in mice.NPJ Sci Food. 2025 Jul 1;9(1):114. doi: 10.1038/s41538-025-00472-1. NPJ Sci Food. 2025. PMID: 40595638 Free PMC article.
Abstract
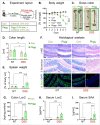
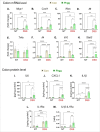
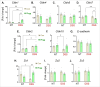
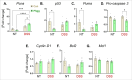
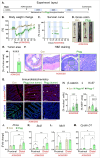
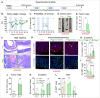
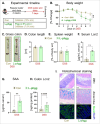
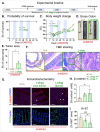
The vital role of naturally occurring dietary fibers (DFs) in maintaining intestinal health has fueled the incorporation of isolated DFs into processed foods. A select group of soluble DFs, such as partially hydrolyzed guar gum (Phgg), are being promoted as dietary supplements to meet recommended DF intake. However, the potential effects of regular consumption of these processed DFs on gastrointestinal health remain largely unknown. The present study assessed the impact of Phgg on the development of intestinal inflammation and colitis-associated colon carcinogenesis (CAC). Wild-type C57BL/6 mice were fed isocaloric diets containing either 7.5% Phgg and 2.5% cellulose (Phgg group) or 10% cellulose (control) for four weeks. To induce colitis, a subgroup of mice from each group was switched to 1.4% dextran sulfate sodium (DSS) in drinking water for seven days. CAC was induced in another subgroup through a single dose of azoxymethane (AOM, 7.5 mg/kg i.p.) followed by three DSS/water cycles. To our surprise, Phgg feeding exacerbated DSS-induced colitis, as evidenced by body weight loss, disrupted colonic crypt architecture, and increased pro-inflammatory markers accompanied by a decrease in anti-inflammatory markers. Additionally, Phgg feeding led to increased colonic expression of genes promoting cell proliferation. Accordingly, extensive colon tumorigenesis was observed in Phgg-fed mice in the AOM/DSS model, whereas the control group exhibited no visible tumors. To investigate whether reducing Phgg has a distinct effect on colitis and CAC development, mice were fed a low-Phgg diet (2.5% Phgg). The low-Phgg group also exhibited increased colitis and tumorigenesis compared to the control, although the severity was markedly lower than in the regular Phgg (7.5%) group, suggesting a dose-dependent effect of Phgg in colitis and CAC development. Our study reveals that Phgg supplementation exacerbates colitis and promotes colon tumorigenesis, warranting further investigation into the potential gastrointestinal health risks associated with processed Phgg consumption.
Keywords: Colorectal cancer; Fermentable dietary fiber; Inflammatory bowel disease; Intestinal inflammation; Oncogenes.
Conflict of interest statement
Declarations Competing interests All authors declare no financial or non-financial competing interests. Additional Declarations: No competing interests reported.
Figures
References
-
- Sung H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 71, 209–249 (2021). - PubMed
-
- Bray F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 74, 229–263 (2024). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

