This is a preprint.
Restoring Mitochondrial Quantity and Quality to Reverse Warburg Effect and Drive Tumor Differentiation
- PMID: 39711563
- PMCID: PMC11661309
- DOI: 10.21203/rs.3.rs-5494402/v1
Restoring Mitochondrial Quantity and Quality to Reverse Warburg Effect and Drive Tumor Differentiation
Update in
-
Restoring mitochondrial quantity and quality to reverse the Warburg effect and drive neuroblastoma differentiation.Proc Natl Acad Sci U S A. 2025 Sep 9;122(36):e2502483122. doi: 10.1073/pnas.2502483122. Epub 2025 Sep 5. Proc Natl Acad Sci U S A. 2025. PMID: 40911595
Abstract
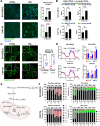
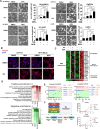
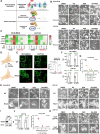
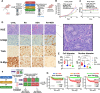
Reduced mitochondrial quality and quantity in tumors is associated with dedifferentiation and increased malignancy. However, it remains unclear how to restore mitochondrial quantity and quality in tumors, and whether mitochondrial restoration can drive tumor differentiation. Our study shows that restoring mitochondrial function using retinoic acid (RA) to boost mitochondrial biogenesis and a mitochondrial uncoupler to enhance respiration synergistically drives neuroblastoma differentiation and inhibits proliferation. U-13C-glucose/glutamine isotope tracing revealed a metabolic shift from the pentose phosphate pathway to oxidative phosphorylation, accelerating the TCA cycle and switching substrate preference from glutamine to glucose. These effects were reversed by ETC inhibitors or in ρ0 cells lacking mtDNA, emphasizing the necessity of mitochondrial function for differentiation. Dietary RA and uncoupler treatment promoted tumor differentiation in an orthotopic neuroblastoma xenograft model, evidenced by neuropil production and Schwann cell recruitment. Single-cell RNA sequencing analysis of the orthotopic xenografts revealed that this strategy effectively eliminated the stem cell population, promoted differentiation, and increased mitochondrial gene signatures along the differentiation trajectory, which could potentially significantly improve patient outcomes. Collectively, our findings establish a mitochondria-centric therapeutic strategy for inducing tumor differentiation, suggesting that maintaining/driving differentiation in tumor requires not only ATP production but also continuous ATP consumption and sustained ETC activity.
Conflict of interest statement
Declaration of interests H.J., Y.L., and J.Y. have been granted a patent related to this manuscript. Additional Declarations: Yes there is potential Competing Interest. H.J., Y.L., and J.Y. have been granted a patent related to this manuscript.
Figures


References
-
- Warburg O. On the origin of cancer cells. Science 123, 309–314 (1956). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

