This is a preprint.
Adolescent-like Processing of Behaviorally Salient Cues in Sensory and Prefrontal Cortices of Adult Preterm-Born Mice
- PMID: 39711564
- PMCID: PMC11661414
- DOI: 10.21203/rs.3.rs-5529783/v1
Adolescent-like Processing of Behaviorally Salient Cues in Sensory and Prefrontal Cortices of Adult Preterm-Born Mice
Abstract
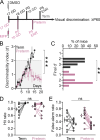
Preterm birth is a leading risk factor for atypicalities in cognitive and sensory processing, but it is unclear how prematurity impacts circuits that support these functions. To address this, we trained adult mice born a day early (preterm mice) on a visual discrimination task and found that they commit more errors and fail to achieve high levels of performance. Using in vivo electrophysiology, we found that the neurons in the primary visual cortex (V1) and the V1-projecting prefrontal anterior cingulate cortex (ACC) are hyper-responsive to the reward, reminiscent of cue processing in adolescence. Moreover, the non-rewarded cue fails to robustly activate the V1 and V1-projecting ACC neurons during error trials, in contrast to prefrontal fast-spiking (FS) interneurons which show elevated error-related activity, suggesting that preterm birth impairs the function of prefrontal circuits for error monitoring. Finally, environmental enrichment, a well-established paradigm that promotes sensory maturation, failed to improve the performance of preterm mice, suggesting limited capacity of early interventions for reducing the risk of cognitive deficits after preterm birth. Altogether, our study for the first time identifies potential circuit mechanisms of cognitive atypicalities in the preterm population and highlights the vulnerability of prefrontal circuits to advanced onset of extrauterine experience.
Keywords: prefrontal; preterm; processing; representation; task; visual.
Figures






References
-
- Perapoch J et al. (2021) Prematurity and ADHD in Childhood: An Observational Register-Based Study in Catalonia. J Atten Disord 25:933–941 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

