The Impact of Piriformospora indica on plant heat and drought tolerance
- PMID: 39711589
- PMCID: PMC11658967
- DOI: 10.3389/fpls.2024.1479561
The Impact of Piriformospora indica on plant heat and drought tolerance
Abstract
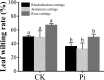
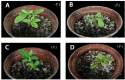
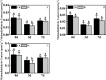
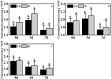
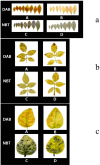
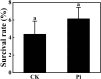
In recent years, the global rise in temperatures has led to drought and heat becoming major environmental stresses that limit plant growth. Previous research has demonstrated the potential of Piriformospora indica in augmenting plant stress resistance. However, specific studies on its effects and underlying mechanisms in cuttings of Rosa chinensis, Jasminum sambac, and Rhododendron simsii Planch are relatively limited. The objective of this study is to explore the effects and mechanisms of P. indica on cuttings and tissue-cultured seedlings of these plants under conditions of drought and high-temperature stress. The experiment involved subjecting P. indica-inoculated and non-inoculated plants to drought (one week without watering) and high-temperature (24-hour exposure to 45°C) stress in a controlled environment chamber. Indicators such as chlorophyll content, chlorophyll fluorescence parameter Fv/Fm, and antioxidant enzyme activity were measured. The results showed that inoculation with P. indica significantly increased the survival rate of the three types of plant cuttings under drought conditions by 13%, 17%, and 16.6% respectively, and resulted in a substantial decrease in malondialdehyde content alongside an increase in chlorophyll content. Under high-temperature stress at 45°C, the chlorophyll fluorescence parameter Fv/Fm values increased by 27.3%, 10.3%, and 51.1% compared to the control group. Furthermore, heat tolerance tests at 42°C showed a 2% higher survival rate in the P. indica inoculated Rhododendron tissue-cultured seedlings than in the control group, with a positive effect observed on the activities of superoxide dismutase and peroxidase. These findings demonstrate that inoculation with P. indica significantly enhances the resistance of Rhododendron, Jasminum sambac, and Rosa to drought and high-temperature stresses, providing insights for sustainable agricultural development and the comprehensive exploitation of the potential value of P. indica.
Keywords: Piriformospora indica; climatic change; drought stress; high-temperature stress; stress resistance.
Copyright © 2024 Ji, Zhang, Huang, Lin, Lu, Wang, Dong, He, Wu and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
An endophytic fungus, Piriformospora indica, enhances drought tolerance of trifoliate orange by modulating the antioxidant defense system and composition of fatty acids.Tree Physiol. 2023 Mar 9;43(3):452-466. doi: 10.1093/treephys/tpac126. Tree Physiol. 2023. PMID: 36263985
-
Exploring rice tolerance to salinity and drought stresses through Piriformospora indica inoculation: understanding physiological and metabolic adaptations.Front Plant Sci. 2024 Sep 24;15:1428631. doi: 10.3389/fpls.2024.1428631. eCollection 2024. Front Plant Sci. 2024. PMID: 39385986 Free PMC article.
-
Effect of the fungus Piriformospora indica on physiological characteristics and root morphology of wheat under combined drought and mechanical stresses.Plant Physiol Biochem. 2017 Sep;118:107-120. doi: 10.1016/j.plaphy.2017.06.005. Epub 2017 Jun 7. Plant Physiol Biochem. 2017. PMID: 28624682
-
The Role of Serendipita indica (Piriformospora indica) in Improving Plant Resistance to Drought and Salinity Stresses.Biology (Basel). 2022 Jun 23;11(7):952. doi: 10.3390/biology11070952. Biology (Basel). 2022. PMID: 36101333 Free PMC article. Review.
-
Molecular mechanism underlying Piriformospora indica-mediated plant improvement/protection for sustainable agriculture.Acta Biochim Biophys Sin (Shanghai). 2019 Mar 1;51(3):229-242. doi: 10.1093/abbs/gmz004. Acta Biochim Biophys Sin (Shanghai). 2019. PMID: 30883651 Review.
References
-
- Abdelaziz M. E., Abdelsattar M., Abdeldaym E. A., Atia M. A., Mahmoud A. W. M., Saad M. M., et al. . (2019). Piriformospora indica alters Na+/K+ homeostasis, antioxidant enzymes and LeNHX1 expression of greenhouse tomato grown under salt stress. Scientia Hortic. 256, 108532. doi: 10.1016/j.scienta.2019.05.059 - DOI
-
- Abdelaziz M. E., Atia M. A., Abdelsattar M., Abdelaziz S. M., Ibrahim T. A., Abdeldaym E. A. (2021). Unravelling the Role of Piriformospora indica in combating water deficiency by modulating physiological performance and chlorophyll metabolism-related genes in Cucumis sativus. Horticulturae 7, 399. doi: 10.3390/horticulturae7100399 - DOI
-
- Aruna S., Rafeekher M., Johnson J., Sarada S., Beena R., Soni K., et al. . (2023). Piriformospora indica Improves Drought Tolerance in Tomato Plants through Enhanced Nutrient Uptake and Antioxidant Enzymes. Int. J. Plant Soil Sci. 35, 865–875. doi: 10.9734/ijpss/2023/v35i183352 - DOI
-
- Atia M. A., Abdeldaym E. A., Abdelsattar M., Ibrahim D. S., Saleh I., Abd Elwahab M., et al. . (2020). Piriformospora indica promotes cucumber tolerance against Root-knot nematode by modulating photosynthesis and innate responsive genes. Saudi J. Biol. Sci. 27, 279–287. doi: 10.1016/j.sjbs.2019.09.007 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

