This is a preprint.
Testosterone Effects on Short-Term Physical, Hormonal, and Neurodevelopmental Outcomes in Infants with 47,XXY/Klinefelter Syndrome: The TESTO Randomized Controlled Trial
- PMID: 39711709
- PMCID: PMC11661422
- DOI: 10.1101/2024.12.09.24318726
Testosterone Effects on Short-Term Physical, Hormonal, and Neurodevelopmental Outcomes in Infants with 47,XXY/Klinefelter Syndrome: The TESTO Randomized Controlled Trial
Update in
-
Testosterone Effects on Short-term Physical, Hormonal, and Neurodevelopmental Outcomes (TESTO) in Infants With 47,XXY.J Clin Endocrinol Metab. 2025 Nov 18;110(12):3493-3504. doi: 10.1210/clinem/dgaf217. J Clin Endocrinol Metab. 2025. PMID: 40177735 Clinical Trial.
Abstract
Context: 47,XXY/Klinefelter syndrome (XXY) is associated with impaired testicular function and differences in physical growth, metabolism, and neurodevelopment. Clinical features of XXY may be attributable to inadequate testosterone during the mini-puberty period of infancy.
Objective: We tested the hypothesis that exogenous testosterone treatment positively effects short-term physical, hormonal, and neurodevelopmental outcomes in infants with XXY.
Design: Double-blind randomized controlled trial, 2017-2021.
Setting: US tertiary care pediatric hospital.
Patients: Infants 30-90 days of age with prenatally identified, non-mosaic 47,XXY (n=71).
Intervention: Testosterone cypionate 25mg intramuscular injections every 4 weeks for 3 doses.
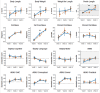
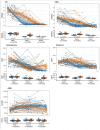
Main outcome measures: The a priori primary outcomes were change in percent fat mass (%FM) z-scores and change in the total composite percentile on Alberta Infant Motor Scales (AIMS) assessment from baseline to 12 weeks.
Results: The between group difference in change in %FM z-scores was -0.57 [95% CI -1.1, -0.06], p=0.03), secondary to greater increases in lean mass in the testosterone-treated group (1.5±0.4 kg vs 1.2±0.4, p=0.001). Testosterone suppressed gonadotropins and inhibin B (p<0.001 for all). In contrast, there were no significant group differences in short term motor, cognitive, or language outcomes (p>0.15 for all).
Conclusions: In this double-blind randomized controlled trial in infants with XXY, testosterone injections resulted in physical effects attributable to systemic androgen exposure; however, there was no impact on neurodevelopmental outcomes and the hypothalamic-pituitary-gonadal axis was suppressed. These results do not support routine testosterone treatment in infants with XXY, however long term follow up on physical health, neurodevelopment and testicular function is needed.
Keywords: Klinefelter syndrome; critical window; mini puberty period; testosterone.
Conflict of interest statement
Conflict of Interest Statement: SD serves as a medical advisor for the non-profit Living with XXY and has received research funding from Association for X&Y Variations (AXYS), Living with XXY, Turner Syndrome Global Alliance, Pediatric Endocrine Society, Boettcher Foundation and the NIH.
Figures

References
-
- Kornman L, Palma-Dias R, Nisbet D, Scott F, Menezes M, da Silva Costa F, McLennan A. Non-Invasive Prenatal Testing for Sex Chromosome Aneuploidy in Routine Clinical Practice. Fetal diagnosis and therapy. 2018;44(2):85–90. - PubMed
-
- Fennoy I. Testosterone and the child (0–12 years) with Klinefelter syndrome (47XXY): a review. Acta paediatrica. 2011;100(6):846–850. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
