This is a preprint.
Dominant negative ADA2 mutations cause ADA2 deficiency in heterozygous carriers
- PMID: 39711711
- PMCID: PMC11661335
- DOI: 10.1101/2024.12.09.24317629
Dominant negative ADA2 mutations cause ADA2 deficiency in heterozygous carriers
Abstract
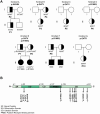
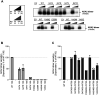
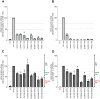
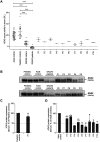
Human ADA2 deficiency (DADA2) is an inborn error of immunity with a broad clinical phenotype which encompasses vasculopathy including livedo racemosa and lacunar strokes, as well as hemato-immunological features. Diagnosis is based on the combination of decreased serum ADA2 activity and the identification of biallelic deleterious alleles in the ADA2 gene. DADA2 carriers harbor a single pathogenic variant in ADA2 and are mostly considered healthy and asymptomatic. However, some DADA2 carriers present a phenotype compatible with DADA2. Here, we report ten patients from seven kindreds presenting with a phenotype indicative of DADA2, in whom only a single pathogenic variant (p.G47R, p.G47V, p.R169Q, p.H424N) was identified. To test whether being heterozygote for specific variants could explain the patients' phenotype, we investigated the effect of the ADA2 missense variants p.G47A, p.G47R, p.G47V, p.G47W, p.R169Q, p.E328K, p.T360A, p.N370K, p.H424N and p.Y453C on ADA2 protein expression, secretion and enzymatic activity. Functional studies indicate that they exert a dominant negative effect on ADA2 enzymatic activity, dimerization and/or secretion. At the molecular level, heterozygosity for these variants mimics what is observed in DADA2. We conclude that humans with heterozygous dominant negative missense variants in ADA2 are at risk of DADA2.
Conflict of interest statement
Conflict-of-interest statement: LRD KU Leuven receives advisory board honorary from Boehringer-Ingelheim and Takeda for IM. IM is the chair of the CSL Behring Chair in Primary Immunodeficiencies, paid to KU Leuven.
Figures

Similar articles
-
Intrinsic Defects in B Cell Development and Differentiation, T Cell Exhaustion and Altered Unconventional T Cell Generation Characterize Human Adenosine Deaminase Type 2 Deficiency.J Clin Immunol. 2021 Nov;41(8):1915-1935. doi: 10.1007/s10875-021-01141-0. Epub 2021 Oct 17. J Clin Immunol. 2021. PMID: 34657246 Free PMC article.
-
Deficiency of Adenosine Deaminase 2 (DADA2): Updates on the Phenotype, Genetics, Pathogenesis, and Treatment.J Clin Immunol. 2018 Jul;38(5):569-578. doi: 10.1007/s10875-018-0525-8. Epub 2018 Jun 27. J Clin Immunol. 2018. PMID: 29951947 Free PMC article. Review.
-
Case Report: Interindividual variability and possible role of heterozygous variants in a family with deficiency of adenosine deaminase 2: are all heterozygous born equals?Front Immunol. 2023 May 3;14:1156689. doi: 10.3389/fimmu.2023.1156689. eCollection 2023. Front Immunol. 2023. PMID: 37207212 Free PMC article.
-
Deficiency of Adenosine Deaminase 2 (DADA2): Hidden Variants, Reduced Penetrance, and Unusual Inheritance.J Clin Immunol. 2020 Aug;40(6):917-926. doi: 10.1007/s10875-020-00817-3. Epub 2020 Jul 8. J Clin Immunol. 2020. PMID: 32638197 Free PMC article.
-
Vasculopathy, Immunodeficiency, and Bone Marrow Failure: The Intriguing Syndrome Caused by Deficiency of Adenosine Deaminase 2.Front Pediatr. 2018 Oct 18;6:282. doi: 10.3389/fped.2018.00282. eCollection 2018. Front Pediatr. 2018. PMID: 30406060 Free PMC article. Review.
References
-
- Navon Elkan P, et al. Mutant Adenosine Deaminase 2 in a Polyarteritis Nodosa Vasculopathy. New England Journal of Medicine. 2014;370(10):921–931. - PubMed
-
- Lee PY, et al. Evaluation and Management of Deficiency of Adenosine Deaminase 2: An International Consensus Statement. JAMA Netw Open. 2023;6(5):e2315894. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
