This is a preprint.
Kir2.1 mutations differentially increase the risk of flecainide proarrhythmia in Andersen Tawil Syndrome
- PMID: 39711719
- PMCID: PMC11661358
- DOI: 10.1101/2024.12.10.24318629
Kir2.1 mutations differentially increase the risk of flecainide proarrhythmia in Andersen Tawil Syndrome
Abstract
Background: Flecainide and other class-Ic antiarrhythmic drugs (AADs) are widely used in Andersen-Tawil syndrome type 1 (ATS1) patients. However, class-Ic drugs might be proarrhythmic in some cases. We investigated the molecular mechanisms of class-I AADs proarrhythmia and whether they might increase the risk of death in ATS1 patients with structurally normal hearts.
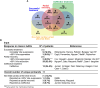
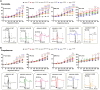
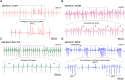
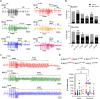
Methods and results: Of 53 ATS1 patients reviewed from the literature, 54% responded partially to flecainide, with ventricular arrhythmia (VA) reduction in only 23%. Of the latter patients, VA persisted in 20-50%. Flecainide was ineffective in 23%, and surprisingly, 13.5% suffered a non-fatal cardiac arrest. In five cardiac-specific ATS1 mouse models (Kir2.1Δ314-315, Kir2.1C122Y, Kir2.1G215D and Kir2.1R67W and Kir2.1S136F), flecainide or propafenone (40 mg/Kg i.p.) differentially prolonged the P wave, and the PR, QRS and QTc intervals compared to Kir2.1WT; Kir2.1S136F had milder effects. Flecainide increased VA inducibility in all mutant mice except Kir2.1S136F, which exhibited significant VA reduction. At baseline, Kir2.1G215D cardiomyocytes had the lowest inward rectifier K+ channel (IK1) reduction, followed by Kir2.1C122Y, Kir2.1R67W and Kir2.1S136F. Kir2.1C122Y cardiomyocytes had a significant decrease in sodium inward current (INa). Flecainide (10 μM) slightly increased IK1 density in Kir2.1WT and Kir2.1S136F, while it decreased both IK1 and INa in Kir2.1C122Y and Kir2.1R67W, despite normal trafficking of mutant channels. Optical mapping in ATS1 patient-specific iPSC-CM monolayers expressing Kir2.1C122Y, Kir2.1G215D and Kir2.1R67W showed an increase in rotor incidence at baseline and under flecainide, confirming the drugś proarrhythmic effect. Lastly, in-silico molecular docking predicts that the Kir2.1-Cys311 pharmacophore-binding site is altered in Kir2.1C122Y heterotetramers, reducing flecainide accessibility and leading to channel closure and arrhythmias.
Conclusions: Class-Ic AADs are only partially effective and might be proarrhythmic in some ATS1 patients. Kir2.1 mutations impacting the resting membrane potential and cellular excitability create a substrate for life-threatening arrhythmias, raising significant concern about using these drugs in some ATS1 patients.
Keywords: ATS1; Arrhythmias; Flecainide; Ion channel diseases; Kir2.1-NaV1.5 channelosome; Sudden Cardiac Death; class-Ic AADs.
Conflict of interest statement
DISCLOSURES None
Figures



Similar articles
-
Extracellular Kir2.1C122Y Mutant Upsets Kir2.1-PIP2 Bonds and Is Arrhythmogenic in Andersen-Tawil Syndrome.Circ Res. 2024 Apr 12;134(8):e52-e71. doi: 10.1161/CIRCRESAHA.123.323895. Epub 2024 Mar 18. Circ Res. 2024. PMID: 38497220 Free PMC article.
-
Extracellular cysteine disulfide bond break at Cys122 disrupts PIP2-dependent Kir2.1 channel function and leads to arrhythmias in Andersen-Tawil Syndrome.bioRxiv [Preprint]. 2023 Jun 8:2023.06.07.544151. doi: 10.1101/2023.06.07.544151. bioRxiv. 2023. PMID: 37333254 Free PMC article. Preprint.
-
Kir2.1 dysfunction at the sarcolemma and the sarcoplasmic reticulum causes arrhythmias in a mouse model of Andersen-Tawil syndrome type 1.Nat Cardiovasc Res. 2022 Oct;1(10):900-917. doi: 10.1038/s44161-022-00145-2. Epub 2022 Oct 17. Nat Cardiovasc Res. 2022. PMID: 39195979 Free PMC article.
-
Kir2.1-NaV1.5 channelosome and its role in arrhythmias in inheritable cardiac diseases.Heart Rhythm. 2024 May;21(5):630-646. doi: 10.1016/j.hrthm.2024.01.017. Epub 2024 Jan 18. Heart Rhythm. 2024. PMID: 38244712 Review.
-
Caveolin-3 Microdomain: Arrhythmia Implications for Potassium Inward Rectifier and Cardiac Sodium Channel.Front Physiol. 2018 Nov 9;9:1548. doi: 10.3389/fphys.2018.01548. eCollection 2018. Front Physiol. 2018. PMID: 30473666 Free PMC article. Review.
References
-
- Priori S. G. et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European heart journal 36, 2793–2867, doi:10.1093/eurheartj/ehv316 (2015). - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
