Comparative efficacy and tolerability of ublituximab vs. other monoclonal antibodies in the treatment of relapsing multiple sclerosis: a systematic review and network meta-analysis of randomized trials
- PMID: 39711787
- PMCID: PMC11659144
- DOI: 10.3389/fneur.2024.1479476
Comparative efficacy and tolerability of ublituximab vs. other monoclonal antibodies in the treatment of relapsing multiple sclerosis: a systematic review and network meta-analysis of randomized trials
Abstract
Background: Relapsing multiple sclerosis (RMS) is a chronic, inflammatory disease of the central nervous system. Ublituximab, an anti-CD20 monoclonal antibody (mAb), is indicated for the treatment of RMS. We performed a systematic literature review (SLR) to identify randomized trials reporting the clinical efficacy and tolerability of ublituximab or comparator disease-modifying therapies (DMTs) for treatment of RMS, and assessed their comparative effects using network meta-analysis (NMA).
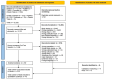
Methods: The SLR involved a comprehensive search across various medical databases to identify relevant studies. Included studies were randomized controlled trials (RCTs) of an adult RMS population, focusing on treatment with at least one of ublituximab, alemtuzumab, natalizumab, ocrelizumab, or ofatumumab. For outcomes included in the NMA (annualized relapse rate (ARR), confirmed disability progression (CDP), and treatment discontinuation rate), rate ratios (RR) or hazard ratios (HR), along with their 95% confidence intervals (CIs), were calculated. We performed NMA using a contrast-based random-effects model within a frequentist framework for all outcomes. Ranking probabilities among comparators, and intervention rankings for the NMA, were estimated using surface under the cumulative ranking curve (SUCRA).
Results: We included 15 RCTs in the review. For the ARR outcome, there was no statistically significant difference between ublituximab and the other included mAbs [ofatumumab (RR 1.02 (95% CI 0.64-1.62)), natalizumab (RR 0.99 (0.59-1.65)), alemtuzumab (RR 0.86 (0.51-1.46)), and ocrelizumab (RR 0.75 (0.44-1.28))]. For CDP at 6 months, our results showed no statistically significant difference between ublituximab and the comparator mAbs [ofatumumab (HR 0.97 (0.49-1.92)), natalizumab (HR 1.13 (0.53-2.40)), alemtuzumab (HR 1.25 (0.56-2.81)), and ocrelizumab (HR 1.29 (0.57-2.90))]. For CDP at 3 and 6 months, there was no statistically significant difference between ublituximab and placebo. The all-cause treatment discontinuation rate analysis showed no significant difference between ublituximab and other mAbs, except for alemtuzumab.
Conclusions: Results of this SLR-informed NMA showed that there is no statistically significant difference between ublituximab and the other mAbs in terms of clinical efficacy. Additionally, the findings show that there is no statistically significant difference in discontinuation rates with the exception of the comparison with alemtuzumab, which may be attributed to its unique dosing schedule.
Keywords: monoclonal antibodies; network meta-analysis; relapsing multiple sclerosis; relapsing-remitting; secondary progressive; systematic review; ublituximab.
Copyright © 2024 Moloney, Mashayekhi, Sharma, Kontogiannis, Ansaripour, Brownlee, Paling and Javanbakht.
Conflict of interest statement
EM, AM, SS, VK, AA, and MJ were employed by Optimax Access Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Nancy Hammond MD. Multiple Sclerosis: Fact, Statistics, and you. (2022). Available at: https://www.healthline.com/health/multiple-sclerosis/facts-statistics-in... (accessed August 30, 2023).
-
- MS in the UK . (2023), p. 1–10. Available at: https://www.mssociety.org.uk/sites/default/files/2022-12/MS%20in%20the%2... (accessed August 30, 2023).
Publication types
LinkOut - more resources
Full Text Sources