Ciprofol Ameliorates Myocardial Ischemia/Reperfusion Injury by Inhibiting Ferroptosis Through Upregulating HIF-1α
- PMID: 39711877
- PMCID: PMC11663391
- DOI: 10.2147/DDDT.S480514
Ciprofol Ameliorates Myocardial Ischemia/Reperfusion Injury by Inhibiting Ferroptosis Through Upregulating HIF-1α
Abstract
Purpose: Ciprofol is a novel intravenous anesthetic that has been increasingly used in clinical anesthesia and sedation. Studies suggested that ciprofol reduced oxidative stress and inflammatory responses to alleviate cerebral ischemia/reperfusion (I/R) injury, but whether ciprofol protects the heart against I/R injury and the mechanisms are unknown. Herein, we assessed the effects of ciprofol on ferroptosis during myocardial I/R injury.
Methods: Experimental models of myocardial I/R injury in mice (ischemia for 30 min and reperfusion for 24 h) and hypoxia/reoxygenation (H/R) injury in H9c2 cardiomyocytes (hypoxia for 6 h followed by 6 h of reoxygenation) were established. Ciprofol was used prior to ischemia or hypoxia. Echocardiography, myocardial TTC staining, HE staining, DAB-enhanced Perl's staining, transmission electron microscopy, FerroOrange staining, Liperfluo staining, JC-1 staining, Rhodamine-123 staining, DCFH-DA staining, and Western blot were performed. Cell viability, serum cardiac enzymes, and oxidative- and ferroptosis-related biomarkers were measured. HIF-1α siRNA transfection and the specific inhibitor BAY87-2243 were utilized for mechanistic investigation.
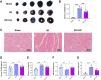
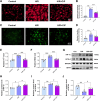
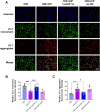
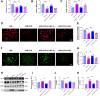
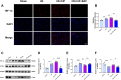
Results: Ciprofol treatment reduced myocardial infarct area and myocardium damage, alleviated oxidative stress and mitochondrial injury, suppressed Fe2+ accumulation and ferroptosis, and improved cardiac function in mice with myocardial I/R injury. Ciprofol also increased cell viability, attenuated mitochondrial damage, and reduced intracellular Fe2+ and lipid peroxidation in cardiomyocytes with H/R injury. Ciprofol enhanced the protein expression of HIF-1α and GPX4 and reduced the expression of ACSL4. Specifically, the protective effects of ciprofol against I/R or H/R injury were abolished by downregulating the expression of HIF-1α using siRNA transfection or the inhibitor BAY87-2243.
Conclusion: Ciprofol ameliorated myocardial I/R injury in mice and H/R injury in cardiomyocytes by inhibiting ferroptosis via the upregulation of HIF-1α expression.
Keywords: GPX4/ACSL4; HIF-1α; ciprofol; ferroptosis; myocardial ischemia/reperfusion injury; oxidative stress.
© 2024 Ding et al.
Conflict of interest statement
The authors declare that they have no competing interests within this work.
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

