From setbacks to success: lessons from the journey of RSV vaccine development
- PMID: 39711948
- PMCID: PMC11660060
- DOI: 10.1177/25151355241308305
From setbacks to success: lessons from the journey of RSV vaccine development
Abstract
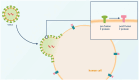
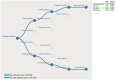
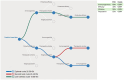
Respiratory syncytial virus (RSV) causes high worldwide infant mortality, as well as a high disease burden in the elderly. Efforts in vaccine development over the past 60 years have recently delivered three approved vaccines and two monoclonal antibodies (mAbs). Looking back at the eventful history of RSV vaccine development, several factors can be identified that have hampered the developmental pathway, including the occurrence of enhanced RSV disease (ERD) in the first vaccine attempt and the difficulty in characterizing and stabilizing the pre-fusion F protein as a vaccine target. Moreover, the need for large trials to test vaccine efficacy, usually done late in development, and the lack of a correlate of protection (CoP) result in significant uncertainties in RSV vaccine development. The use of controlled human infection models (CHIMs) may provide a solution for some of these problems: through swift, cost-efficient and closely monitored assessment of vaccine safety and efficacy in early clinical phases, vaccines can either 'fail fast' or show results supporting further investments. Moreover, CHIMs facilitate the assessment of disease and could assist in the identification of a CoP supporting late-stage development. Although some factors may affect translatability to real-world vaccine efficacy, CHIMs can support the clinical development pathway in various ways. We advocate for, and demonstrate, a conceptual and rational design of RSV vaccine development. Assessing protective efficacy early on would result in the most cost-efficient pathway and identification of target populations should be done as early as possible. For RSV, elderly individuals and people in low- and middle-income countries are high-impact populations for RSV prevention. While RSV immunization is now available in certain regions, global access is not accomplished yet, and worldwide prevention does not seem within reach. Quick and cost-effective assessments of candidates currently in the pipeline could contribute to future successes in the battle against RSV.
Keywords: RSV; clinical development; controlled human infection model; therapeutic; vaccine.
Plain language summary
From setbacks to success: lessons from the journey of RSV vaccine development Respiratory syncytial virus (RSV) leads to the deaths of many young children worldwide, as well as severe infections and deaths in the elderly. The search for an effective vaccine has lasted over 60 years, in which many vaccine candidates were developed and tested. Only recently, three vaccines and two monoclonal antibodies were approved for medical use, to prevent disease in newborns, pregnant individuals and the elderly. Several lessons can be learned from the long and difficult journey of RSV vaccine development. The efficacy of a vaccine is often only studied in large trials, sometimes with over 10 000 participants; this could also be done, however, in smaller studies in which participants receive the vaccine candidate and are given the virus under controlled conditions. Though these smaller studies may not substitute the larger one, they can still save time and money, and provide more information about RSV disease and how the immune system battles the virus. Many RSV vaccine candidates are still being developed; we advocate for and demonstrate a thoughtful design of the steps to test these vaccines, using the controlled infection studies to test in a time- and cost-efficient manner.
© The Author(s), 2024.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

