Gene expression levels associated with impaired immune response and increased proliferation could serve as biomarkers for women following cervical cancer screening programmes
- PMID: 39712018
- PMCID: PMC11659148
- DOI: 10.3389/fimmu.2024.1507193
Gene expression levels associated with impaired immune response and increased proliferation could serve as biomarkers for women following cervical cancer screening programmes
Abstract
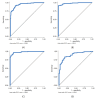
Human papilloma virus (HPV) infections vary in their oncogenic potential, and whether an infection progresses to cervical intraepithelial neoplasia (CIN) also depends on the immune response. Therefore, the aim of the present study was to explore biomarkers related to the immune system and cell proliferation, in combination with HPV classified as having high (HOP) or low oncogenic potential (LOP), that can possibly guide a more accurate identification of women following cervical cancer screening programmes in need for immediate follow-up with a biopsy. A next-generation sequencing transcriptomic immune profile analysis applied to 28 persistent CIN3 lesions and 14 normal biopsies identified four genes, the immune markers ARG1 and HLA-DQB2 and the tumour markers CDKN2A and KRT7, as possible markers for differentiating between CIN3 and normal tissue. To validate these findings, analysis of the relative gene expression of these markers by use of reverse transcriptase real-time quantitative polymerase chain reaction was performed in an independent cohort of 264 (82 normal, 64 CIN1, and 118 CIN2/CIN3) biopsies, and the data were combined with information on the HOP- or LOP-HPV identified in the biopsies. Statistical analysis was performed with receiver operating characteristic curves, reporting area under the curve (AUC) with 95% confidence intervals (CIs), and logistic regression. Statistically significantly higher median expression levels of CDKN2A (p < 0.001) and KRT7 (p = 0.045) and significantly lower expression of ARG1 (p = 0.012) were found in biopsies with HOP-HPV infections, with no difference detected for HLA-DQB2 (p = 0.82). Models using expression levels of CDKN2A (AUC, 0.91; 95% CI, 0.86-0.95), KRT7 (0.86, 0.81-0.91), or ARG1 (0.78, 0.70-0.85) together with HOP/LOP-HPV class were significantly better than HPV class alone (0.72, 0.66-0.79) in discriminating CIN2/3 versus CIN1 (p < 0.001, p < 0.001, and p = 0.014, respectively).
Keywords: HPV; NGS; ROC-AUC; biomarkers; cervical precancer; immunology; qPCR.
Copyright © 2024 Ovestad, Dalen, Soreng, Akbari, Lapin, Janssen, Austdal, Munk and Gudlaugsson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinogenic Risks Humans. (2012) 100(Pt B):1–441. Available online at: https://publications.iarc.fr/119. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

