Autologous porcine VRAM flap model for VCA research
- PMID: 39712036
- PMCID: PMC11659244
- DOI: 10.3389/frtra.2024.1504959
Autologous porcine VRAM flap model for VCA research
Abstract
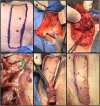
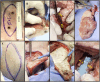
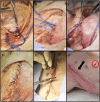
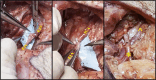
Introduction: As research advances in vascularized composite allotransplantation (VCA), large animal models are essential for translational studies related to immune rejection and graft survival. However, procurement of large flaps can cause significant defects, complicating wound closure and increasing postoperative risks. This study details the surgical techniques and outcomes of autologous vertical rectus abdominis myocutaneous (VRAM) flap transplantation and neck flap isolation with induced ischemia in a swine model. The purpose of this study was to identify the most effective control procedure for use in future VRAM flap allotransplantation research.
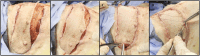
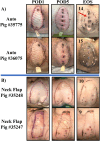
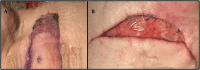
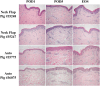
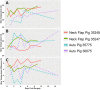
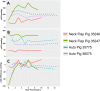
Methods: We performed two left heterotopic autologous VRAM flap transplants and two right anterolateral neck flap isolations using female Yucatan pigs. Postoperatively, animals were monitored for complications and flap healing, with punch biopsies taken on POD1, 5, and at the end of the study for histological analysis. Transcutaneous oxygen and temperature were also recorded.
Results: Both autologous flaps survived after vessel anastomosis, with effective closure of abdominal defects using suturable mesh, and no postoperative complications were observed. Histology revealed mild dermal edema and perivascular inflammation on POD5. In the neck flap group, both flaps survived temporary ischemia, however, postoperative complications included dorsal flap necrosis and wound dehiscence, requiring reoperation. No gross inflammation or edema was observed following surgery and histologically there was only mild dermal edema on POD5.
Discussion: We have developed a low-risk, technically feasible porcine autologous VRAM flap transplantation model and our findings support its use in future VCA studies.
Keywords: autologous; ischemia; neck flap; swine; vertical rectus abdominis myocutaneous flap.
© 2024 Blades, Dumanian, Wang, Wang, Li, Washington, Slade, Evans, Arrowsmith, Farkash, Yu, Greyson, Huang, Navarro-Alvarez and Mathes.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Heterotopic Transplantation of Allogeneic Vertical Rectus Abdominis Myocutaneous Flaps in Miniature Swine.J Surg Res. 2020 Oct;254:175-182. doi: 10.1016/j.jss.2020.04.026. Epub 2020 May 22. J Surg Res. 2020. PMID: 32450418
-
Development of a Porcine VCA Model Using an External Iliac Vessel-Based Vertical Rectus Abdominus Myocutaneous Flap.J Reconstr Microsurg. 2025 May;41(4):339-346. doi: 10.1055/s-0044-1788812. Epub 2024 Aug 6. J Reconstr Microsurg. 2025. PMID: 39106899
-
Use of adjuvant techniques improves surgical outcomes of complex vertical rectus abdominis myocutaneous flap reconstructions of pelvic cancer defects.Plast Reconstr Surg. 2011 Aug;128(2):447-458. doi: 10.1097/PRS.0b013e31821e6fd2. Plast Reconstr Surg. 2011. PMID: 21788836
-
Vertical Rectus Abdominis Myocutaneous Versus Alternative Flaps for Perineal Repair After Abdominoperineal Excision of the Rectum in the Era of Laparoscopic Surgery.Ann Plast Surg. 2017 Jul;79(1):101-106. doi: 10.1097/SAP.0000000000001137. Ann Plast Surg. 2017. PMID: 28542071 Review.
-
Vertical rectus abdominis flap (VRAM) for perineal reconstruction following pelvic surgery: A systematic review.J Plast Reconstr Aesthet Surg. 2021 Mar;74(3):523-529. doi: 10.1016/j.bjps.2020.10.100. Epub 2020 Nov 9. J Plast Reconstr Aesthet Surg. 2021. PMID: 33317983
References
-
- Wahyudi M, Satria O, Aprilya D, Nong I. Vertical rectus abdominis myocutaneous flap for reconstruction of forequarter amputation defect after shoulder soft tissue sarcoma resection: technical consideration. Plast Reconstr Surg Glob Open. (2023) 11(6):e5077. 10.1097/GOX.0000000000005077 - DOI - PMC - PubMed
-
- Salo J, Tukiainen E. Flap reconstruction of the chest wall after oncologic resection. Curr Chall Thorac Surg. (2020) 2:5. 10.21037/ccts.2019.12.05 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

