N, N'-Di-benzyl-ethyl-enedi-ammonium dichloride
- PMID: 39712151
- PMCID: PMC11660484
- DOI: 10.1107/S205698902400954X
N, N'-Di-benzyl-ethyl-enedi-ammonium dichloride
Abstract
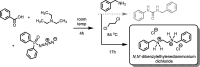
The isolation and crystalline structure of N,N'-di-benzyl-ethyl-enedi-ammonium dichloride, C16H22N2 2+·2Cl-, is reported. This was obtained as an unintended product of an attempted Curtius rearrangement that involved benzyl-amine as one of the reagents and 1,2-di-chloro-ethane as the solvent. Part of a series of reactions of a course-based undergraduate research experience (CURE), this was not the intended reaction outcome. The goal of the course was to engage students as active participants in a laboratory experience which applies the foundational techniques of a synthetic organic laboratory, using the Curtius rearrangement as a tool for the assembly of medicinally significant scaffolds. The isolation of the title compound, N,N'-di-benzyl-ethyl-enedi-ammonium dichloride, the result of the 1,2-di-chloro-ethane solvent outcompeting the Curtius iso-cyanate inter-mediate in the reaction with the nucleophilic amine, confirms the importance of conducting research at the undergraduate level where the outcome is not predetermined. The solid-state structure of N,N'-di-benzyl-ethyl-enedi-ammonium dichloride was found to feature an all-trans methyl-ene-ammonium backbone. Strong N-H⋯Cl hydrogen bonds and C-H⋯Cl inter-actions lead to a layered structure with pseudo-translational symmetry emulating a C-centered setting. Different phenyl torsion angles at each end of the mol-ecule enable a more stable packing by allowing stronger hydrogen-bonding inter-actions, leading to a more ordered but lower symmetry and modulated structure in P21/n.
Keywords: crystal structure; hydrogen bonding; modulation; pseudo-translation; side reaction.
© Marmande et al. 2024.
Figures
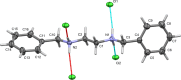
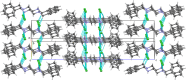
 0] (the modulation direction). 50% probability ellipsoids.
0] (the modulation direction). 50% probability ellipsoids.
References
-
- Asadi, H., Golchoubia, H. & Welter, R. (2005). J. Mol. Struct.779, 30–37.
-
- Brownell, S. E. & Kloser, M. J. (2015). Stud. High. Educ.40, 525–544.
-
- Bruker (2022). APEX4 and SAINT. Bruker AXS Inc. Madison, Wisconsin, USA.
-
- Curtius, T. (1890). Ber. Deutsch. Chem. Ges. zu Berlin, 23(2), 3023–3033.
-
- Curtius, T. (1894). J. Prakt. Chem.50, 275–294.
LinkOut - more resources
Full Text Sources
