Crystal structures and photophysical properties of mono- and dinuclear ZnII complexes flanked by tri-ethyl-ammonium
- PMID: 39712172
- PMCID: PMC11660477
- DOI: 10.1107/S2056989024010302
Crystal structures and photophysical properties of mono- and dinuclear ZnII complexes flanked by tri-ethyl-ammonium
Abstract
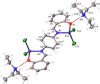
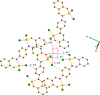
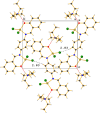
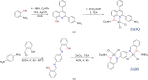
Two new zinc(II) complexes, tri-ethyl-ammonium di-chlorido-[2-(4-nitro-phen-yl)-4-phenyl-quinolin-8-olato]zinc(II), (C6H16N){Zn(C21H13N2O3)Cl2] (ZnOQ), and bis-(tri-ethyl-ammonium) {2,2'-[1,4-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato}bis-[di-chlorido-zinc(II)], (C6H16N)2[Zn2(C20H14N2O2)Cl4] (ZnBS), were synthesized and their structures were determined using ESI-MS spectrometry, 1H NMR spectroscopy, and single-crystal X-ray diffraction. The results showed that the ligands 2-(4-nitro-phen-yl)-4-phenyl-quinolin-8-ol (HOQ) and N,N'-bis-(2-hy-droxy-benzyl-idene)benzene-1,4-di-amine (H2BS) were deprotonated by tri-ethyl-amine, forming the counter-ion Et3NH+, which inter-acts via an N-H⋯O hydrogen bond with the ligand. The ZnII atoms have a distorted trigonal-pyramidal (ZnOQ) and distorted tetra-hedral (ZnBS) geometries with a coord-ination number of four, coordinating with the ligands via N and O atoms. The N atoms coordinating with ZnII correspond to the heterocyclic nitro-gen for the HOQ ligand, while for the H2BS ligand, it is the nitro-gen of the imine (CH=N). The crystal packing of ZnOQ is characterized by C-H⋯π inter-actions, while that of ZnBS by C-H⋯Cl inter-actions. The emission spectra showed that ZnBS complex exhibits green fluorescence in the solid state with a small band-gap energy, and the ZnOQ complex does exhibit non-fluorescence.
Keywords: 8-hydroxyquinoline derivatives; Schiff base.; Zn(II) complex; crystal structure.
© Le Thi Hong et al. 2024.
Figures




Similar articles
-
Crystal structure of (μ2-7-{[bis-(pyridin-2-ylmeth-yl)amino-1κ3 N,N',N'']meth-yl}-5-chloro-quinolin-8-olato-2κN;1:2κ2 O)tri-chlorido-1κCl,2κ2 Cl-dizinc(II).Acta Crystallogr E Crystallogr Commun. 2024 Oct 15;80(Pt 11):1175-1179. doi: 10.1107/S2056989024009782. eCollection 2024 Oct 1. Acta Crystallogr E Crystallogr Commun. 2024. PMID: 39712167 Free PMC article.
-
Geometrical variations of two manganese(II) complexes with closely related quinoline-based tripodal ligands.Acta Crystallogr E Crystallogr Commun. 2021 Sep 28;77(Pt 10):982-988. doi: 10.1107/S2056989021009786. eCollection 2021 Oct 1. Acta Crystallogr E Crystallogr Commun. 2021. PMID: 34667623 Free PMC article.
-
Crystal structures of two CuII compounds: catena-poly[[chlorido-copper(II)]-μ-N-[eth-oxy(pyridin-2-yl)methyl-idene]-N'-[oxido(pyridin-3-yl)methyl-idene]hydrazine-κ4 N,N',O:N''] and di-μ-chlorido-1:4κ2 Cl:Cl-2:3κ2 Cl:Cl-di-chlorido-2κCl,4κCl-bis[μ3-eth-oxy(pyridin-2-yl)methano-lato-1:2:3κ3 O:N,O:O;1:3:4κ3 O:O:N,O]bis-[μ2-eth-oxy(pyridin-2-yl)methano-lato-1:2κ3 N,O:O;3:4κ3 N,O:O]tetracopper(II).Acta Crystallogr E Crystallogr Commun. 2019 Jun 28;75(Pt 7):1069-1075. doi: 10.1107/S2056989019008922. eCollection 2019 Jul 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 31392027 Free PMC article.
-
The cocrystal aqua-chlorido{6,6'-di-tert-butyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}-manganese(III)-chlorido{6,6'-di-tert-butyl-2,2'-[1,2-phenyl-enebis(nitrilo-methyl-idyne)]diphenolato-κO,N,N',O'}(methanol-κO)manganese(III) (1/1).Acta Crystallogr Sect E Struct Rep Online. 2008 Apr 4;64(Pt 5):m626-7. doi: 10.1107/S1600536808006818. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202181 Free PMC article.
-
Di-aqua-bis-{μ-1,5-bis-[(pyridin-2-yl)methyl-idene]carbonohydrazide(1-)}di-μ-chlorido-tetra-chlorido-tetra-zinc(II).Acta Crystallogr E Crystallogr Commun. 2020 Jul 24;76(Pt 8):1349-1352. doi: 10.1107/S2056989020009834. eCollection 2020 Aug 1. Acta Crystallogr E Crystallogr Commun. 2020. PMID: 32844027 Free PMC article.
References
-
- Das, M. K. & Ghosh, S. (1998). Indian J. Chem.3, 272–275.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
-
- Du, L., Zhang, T., Huang, X., Xu, Y., Tan, M., Huang, Y., Chen, Y. & Qin, Q. (2023). Dalton Trans.52, 4737–4751. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
