Bioinformatics-Facilitated Identification of Novel Bacterial Sulfoglycosidases That Hydrolyze 6-Sulfo- N-acetylglucosamine
- PMID: 39712202
- PMCID: PMC11659886
- DOI: 10.1021/acsbiomedchemau.4c00088
Bioinformatics-Facilitated Identification of Novel Bacterial Sulfoglycosidases That Hydrolyze 6-Sulfo- N-acetylglucosamine
Abstract
Glycan sulfation is a widespread postglycosylation modification crucial for modulating biological functions including cellular adhesion, signaling, and bacterial colonization. 6-Sulfo-β-GlcNAcases are a class of enzyme that alters sulfation patterns. Such changes in sulfation patterns are linked to diseases such as bowel inflammation, colitis, and cancer. Despite their significance, 6-sulfo-β-GlcNAcases, which cleave β-linked 6-sulfo-N-acetylglucosamine (6S-GlcNAc), have been but rarely identified. This scarcity results mainly from the short, diverse, and distinctive sulfate-binding motifs required for recognition of the 6-sulfate group in 6S-GlcNAc in addition to the conserved GH20 family features. In this study, we discovered 6-sulfo-β-GlcNAcases and assigned two novel sulfate-binding motifs by the use of comparative genomics, structural predictions, and activity-based screening. Our findings expand the known microbiota capable of degrading sulfated glycans and add significant enzymes to the tool kit for analysis and synthesis of sulfated oligosaccharides.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
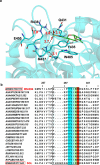
Figures



References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
