Bacterial Cytochrome P450 Catalyzed Macrocyclization of Ribosomal Peptides
- PMID: 39712204
- PMCID: PMC11659900
- DOI: 10.1021/acsbiomedchemau.4c00080
Bacterial Cytochrome P450 Catalyzed Macrocyclization of Ribosomal Peptides
Abstract
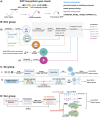
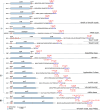
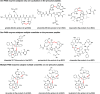
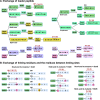
Macrocyclization is a vital process in the biosynthesis of ribosomally synthesized and post-translationally modified peptides (RiPPs), significantly enhancing their structural diversity and biological activity. Universally found in living organisms, cytochrome P450 enzymes (P450s) are versatile catalysts that facilitate a wide array of chemical transformations and have recently been discovered to contribute to the expansion and complexity of the chemical spectrum of RiPPs. Particularly, P450-catalyzed biaryl-bridged RiPPs, characterized by highly modified structures, represent an intriguing but underexplored class of natural products, as demonstrated by the recent discovery of tryptorubin A, biarylitide and cittilin. These P450 enzymes demonstrate their versatility by facilitating peptide macrocyclization through the formation of carbon-carbon (C-C), carbon-nitrogen (C-N) and ether bonds between the side chains of tyrosine (Tyr), tryptophan (Trp) and histidine (His). This Review briefly highlights the latest progress in P450-catalyzed macrocyclization within RiPP biosynthesis, resulting in the generation of structurally complex RiPPs. These findings have expedited the discovery and detailed analysis of new P450s engaged in RiPP biosynthetic pathways.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Bacterial Cytochrome P450 Catalyzed Post-translational Macrocyclization of Ribosomal Peptides.Angew Chem Int Ed Engl. 2023 Nov 13;62(46):e202311533. doi: 10.1002/anie.202311533. Epub 2023 Oct 13. Angew Chem Int Ed Engl. 2023. PMID: 37767859
-
Cytochromes P450 involved in bacterial RiPP biosyntheses.J Ind Microbiol Biotechnol. 2023 Feb 17;50(1):kuad005. doi: 10.1093/jimb/kuad005. J Ind Microbiol Biotechnol. 2023. PMID: 36931895 Free PMC article. Review.
-
Exploring the Diverse Landscape of Biaryl-Containing Peptides Generated by Cytochrome P450 Macrocyclases.J Am Chem Soc. 2023 Oct 11;145(40):22047-22057. doi: 10.1021/jacs.3c07140. Epub 2023 Sep 27. J Am Chem Soc. 2023. PMID: 37756205
-
Cytochromes P450 Associated with the Biosyntheses of Ribosomally Synthesized and Post-translationally Modified Peptides.ACS Bio Med Chem Au. 2023 Jul 13;3(5):371-388. doi: 10.1021/acsbiomedchemau.3c00026. eCollection 2023 Oct 18. ACS Bio Med Chem Au. 2023. PMID: 37876494 Free PMC article. Review.
-
Genome-Guided Discovery of the First Myxobacterial Biarylitide Myxarylin Reveals Distinct C-N Biaryl Crosslinking in RiPP Biosynthesis.Molecules. 2021 Dec 10;26(24):7483. doi: 10.3390/molecules26247483. Molecules. 2021. PMID: 34946566 Free PMC article.
Cited by
-
The Deep Mining Era: Genomic, Metabolomic, and Integrative Approaches to Microbial Natural Products from 2018 to 2024.Mar Drugs. 2025 Jun 23;23(7):261. doi: 10.3390/md23070261. Mar Drugs. 2025. PMID: 40710486 Free PMC article. Review.
-
P450 cyptide synthase MpoB catalyzes the cross-linking of the YPW motif on the precursor peptide.RSC Chem Biol. 2025 Jul 24. doi: 10.1039/d5cb00153f. Online ahead of print. RSC Chem Biol. 2025. PMID: 40718713 Free PMC article.
-
Biosynthesis of Biphenomycin-like Macrocyclic Peptides by Formation and Cross-Linking of Ortho-Tyrosines.J Am Chem Soc. 2025 Jul 9;147(27):23781-23796. doi: 10.1021/jacs.5c06044. Epub 2025 Jun 26. J Am Chem Soc. 2025. PMID: 40568902 Free PMC article.
-
P450 Biarylitide Synthase ShyB Catalyzes the Cross-Link Between His-C2 and Tyr-O4 on the Precursor Peptide.Org Lett. 2025 Jun 6;27(22):5715-5719. doi: 10.1021/acs.orglett.5c01487. Epub 2025 May 28. Org Lett. 2025. PMID: 40436370 Free PMC article.
-
Phylogeny-guided discovery of a promiscuous P450 macrocyclase for the production of diverse atropopeptides.Chem Sci. 2025 Aug 6. doi: 10.1039/d5sc03525b. Online ahead of print. Chem Sci. 2025. PMID: 40822110 Free PMC article.
References
-
- Moyer T. B.; Parsley N. C.; Sadecki P. W.; Schug W. J.; Hicks L. M. Leveraging orthogonal mass spectrometry based strategies for comprehensive sequencing and characterization of ribosomal antimicrobial peptide natural products. Nat. Prod Rep 2021, 38 (3), 489–509. 10.1039/D0NP00046A. - DOI - PMC - PubMed
-
- Imai Y.; Meyer K. J.; Iinishi A.; Favre-Godal Q.; Green R.; Manuse S.; Caboni M.; Mori M.; Niles S.; Ghiglieri M.; Honrao C.; Ma X.; Guo J. J.; Makriyannis A.; Linares-Otoya L.; Bohringer N.; Wuisan Z. G.; Kaur H.; Wu R.; Mateus A.; Typas A.; Savitski M. M.; Espinoza J. L.; O’Rourke A.; Nelson K. E.; Hiller S.; Noinaj N.; Schaberle T. F.; D’Onofrio A.; Lewis K. A new antibiotic selectively kills Gram-negative pathogens. Nature 2019, 576 (7787), 459–464. 10.1038/s41586-019-1791-1. - DOI - PMC - PubMed
-
- Culvenor C.; Beck A.; Clarke M.; Cockrum P.; Edgar J.; Frahn J.; Jago M.; Lanigan G.; Payne A.; Peterson J.; et al. Isolation of toxic metabolites of Phomopsis leptostromiformis responsible for lupinosis. Australian Journal of Biological Sciences 1977, 30 (4), 269–278. 10.1071/BI9770269. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
