Design, Synthesis, and Evaluation of Trihalomethyl Ketone Derivatives of Neocarzilin A as Improved Antimetastatic Agents
- PMID: 39712208
- PMCID: PMC11659896
- DOI: 10.1021/acsbiomedchemau.4c00087
Design, Synthesis, and Evaluation of Trihalomethyl Ketone Derivatives of Neocarzilin A as Improved Antimetastatic Agents
Abstract
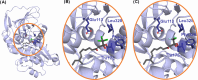
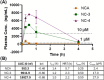
Vesicle Amine Transport-1 (VAT1) is a protein that is overexpressed in many cancers, including breast cancer, glioblastoma, and angiosarcoma. High VAT1 expression correlates with poor overall survival, and genetic knockout models of VAT1 indicate potent antimigratory activity, suggesting that VAT1 is a promising antimetastasis target. Recently, the natural product neocarzilin A (NCA) from Streptomyces carzinostaticus was reported to be the first validated small-molecule inhibitor of VAT1, having strong activity in metastasis models of angiosarcoma and breast cancer. While knockdown of VAT1 has no effect on cell viability, NCA has significant cytotoxicity, suggesting that NCA is not selective for VAT1. Additionally, NCA has poor aqueous solubility, making in vivo administration of NCA challenging and thus limiting its therapeutic potential. Here, we report the design, synthesis, bioactivity, and pharmacokinetics of novel NCA derivatives with improved drug-like properties. Specifically, we have developed derivatives with altered warheads, replacing chlorines on the trichloroketone with fluorines. Using a modified synthetic route, we accessed NCA derivatives with greater than 25-fold improvements in solubility and 30-fold improvements in the antimigratory to antiproliferative bioactivity ratio. The two best derivatives, along with the parent, were analyzed for oral bioavailability, with the two more soluble derivatives showing greatly improved bioavailability. Overall, these studies have resulted in the development of VAT1 inhibitors with improved properties, which will enable further study of the pharmacological inhibition of VAT1 as an antimetastatic strategy. Additionally, these studies provide insights into novel trihalomethyl ketone warheads and identify chlorodifluoroketone as a potent and selective new warhead.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
LinkOut - more resources
Full Text Sources
