Brain fingerprint and subjective mood state across the menstrual cycle
- PMID: 39712222
- PMCID: PMC11659225
- DOI: 10.3389/fnins.2024.1432218
Brain fingerprint and subjective mood state across the menstrual cycle
Abstract
Background: Brain connectome fingerprinting represents a recent and valid approach in assessing individual identifiability on the basis of the subject-specific brain functional connectome. Although this methodology has been tested and validated in several neurological diseases, its performance, reliability and reproducibility in healthy individuals has been poorly investigated. In particular, the impact of the changes in brain connectivity, induced by the different phases of the menstrual cycle (MC), on the reliability of this approach remains unexplored. Furthermore, although the modifications of the psychological condition of women during the MC are widely documented, the possible link with the changes of brain connectivity has been poorly investigated.
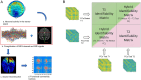
Methods: We conducted the Clinical Connectome Fingerprint (CCF) analysis on source-reconstructed magnetoencephalography signals in a cohort of 24 women across the MC.
Results: All the parameters of identifiability did not differ according to the MC phases. The peri-ovulatory and mid-luteal phases showed a less stable, more variable over time, brain connectome compared to the early follicular phase. This difference in brain connectome stability in the alpha band significantly predicted the self-esteem level (p-value <0.01), mood (p-value <0.01) and five (environmental mastery, personal growth, positive relations with others, purpose in life, and self-acceptance) of the six dimensions of well-being (p-value <0.01, save autonomy).
Conclusion: These results confirm the high reliability of the CCF as well as its independence from the MC phases. At the same time the study provides insights on changes of the brain connectome in the different phases of the MC and their possible role in affecting women's subjective mood state across the MC. Finally, these changes in the alpha band share a predictive power on self-esteem, mood and well-being.
Keywords: brain connectivity; brain fingerprint; depression; menstrual cycle; self-esteem; well-being.
Copyright © 2024 Cipriano, Liparoti, Troisi Lopez, Romano, Sarno, Mazzara, Alivernini, Lucidi, Sorrentino and Sorrentino.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Uncovering longitudinal changes in the brain functional connectome along the migraine cycle: a multilevel clinical connectome fingerprinting framework.J Headache Pain. 2025 Feb 10;26(1):29. doi: 10.1186/s10194-025-01969-6. J Headache Pain. 2025. PMID: 39930372 Free PMC article.
-
Reduced clinical connectome fingerprinting in multiple sclerosis predicts fatigue severity.Neuroimage Clin. 2023;39:103464. doi: 10.1016/j.nicl.2023.103464. Epub 2023 Jun 28. Neuroimage Clin. 2023. PMID: 37399676 Free PMC article.
-
Functional brain network topology across the menstrual cycle is estradiol dependent and correlates with individual well-being.J Neurosci Res. 2021 Sep;99(9):2271-2286. doi: 10.1002/jnr.24898. Epub 2021 Jun 10. J Neurosci Res. 2021. PMID: 34110041 Free PMC article.
-
The Prevalence of Menstrual Cycle Disorders and Menstrual Cycle-Related Symptoms in Female Athletes: A Systematic Literature Review.Sports Med. 2023 Oct;53(10):1963-1984. doi: 10.1007/s40279-023-01871-8. Epub 2023 Jun 30. Sports Med. 2023. PMID: 37389782
-
Physiological Changes in Women's Skin During the Menstrual Cycle: A Scoping Review.Cureus. 2024 Dec 7;16(12):e75286. doi: 10.7759/cureus.75286. eCollection 2024 Dec. Cureus. 2024. PMID: 39776723 Free PMC article.
Cited by
-
Uncovering longitudinal changes in the brain functional connectome along the migraine cycle: a multilevel clinical connectome fingerprinting framework.J Headache Pain. 2025 Feb 10;26(1):29. doi: 10.1186/s10194-025-01969-6. J Headache Pain. 2025. PMID: 39930372 Free PMC article.
References
-
- Avila-Varela D. S., Hidalgo-Lopez E., Dagnino P. C., Acero-Pousa I., del Agua E., Deco G., et al. . (2024). Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women. npj Women’s Heal. 2, 1–9. doi: 10.1038/s44294-024-00012-4 - DOI
LinkOut - more resources
Full Text Sources

