The pathogenesis of gout
- PMID: 39712248
- PMCID: PMC11659655
- DOI: 10.4078/jrd.2024.0054
The pathogenesis of gout
Abstract
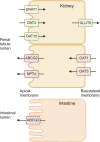
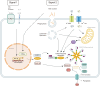
Gout is the most common inflammatory arthritis in adults, associated with hyperuricemia and the chronic deposition of monosodium urate (MSU) crystals. Hyperuricemia results from increased production of uric acid and decreased excretion by the kidneys and intestines. Urate excretion is regulated by a group of urate transporters, and decreased renal or intestinal excretion is the primary mechanism of hyperuricemia in most people. Genetic variability in these urate transporters is strongly related to variances in serum urate levels. Not all individuals with hyperuricemia show deposition of MSU crystals or develop gout. The initiation of the inflammatory response to MSU crystals is mainly mediated by the nucleotide-binding oligomerization domain-, leucine-rich repeat- and pyrin domain-containing protein 3 (NLRP3) inflammasome. The activated NLRP3 inflammasome complex cleaves pro-interleukin-1β (IL-1β) into its active form, IL-1β, which is a key mediator of the inflammatory response in gout. IL-1β leads to the upregulation of cytokines and chemokines, resulting in the recruitment of neutrophils and other immune cells. Neutrophils recruited to the site of inflammation also play a role in resolving inflammation. Aggregated neutrophil extracellular traps (NETs) trap and degrade cytokines and chemokines through NET-bound proteases, promoting the resolution of inflammation. Advanced gout is characterized by tophi, chronic inflammatory responses, and structural joint damage. Tophi are chronic foreign body granuloma-like structures containing collections of MSU crystals encased by inflammatory cells and connective tissue. Tophi are closely related to chronic inflammation and structural damage.
Keywords: Gout; Inflammasomes; Pathogenesis; Uric acid.
Copyright © 2025 by The Korean College of Rheumatology. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
-
Pathophysiology of Gout.Semin Nephrol. 2020 Nov;40(6):550-563. doi: 10.1016/j.semnephrol.2020.12.001. Semin Nephrol. 2020. PMID: 33678310 Review.
-
NLRP3 inflammasome-mediated neutrophil recruitment and hypernociception depend on leukotriene B(4) in a murine model of gout.Arthritis Rheum. 2012 Feb;64(2):474-84. doi: 10.1002/art.33355. Arthritis Rheum. 2012. PMID: 21952942
-
A wild rice-derived peptide R14 ameliorates monosodium urate crystals-induced IL-1β secretion through inhibition of NF-κB signaling and NLRP3 inflammasome activation.PeerJ. 2023 May 12;11:e15295. doi: 10.7717/peerj.15295. eCollection 2023. PeerJ. 2023. PMID: 37197585 Free PMC article.
-
Macrophage-derived IL-1β enhances monosodium urate crystal-triggered NET formation.Inflamm Res. 2017 Mar;66(3):227-237. doi: 10.1007/s00011-016-1008-0. Epub 2016 Nov 16. Inflamm Res. 2017. PMID: 27853847 Free PMC article.
Cited by
-
Misdiagnosis of sacroiliac joint gout as ankylosing spondylitis: Solving the diagnostic dilemma with dual-energy computed tomography.SAGE Open Med Case Rep. 2025 Jun 20;13:2050313X251351769. doi: 10.1177/2050313X251351769. eCollection 2025. SAGE Open Med Case Rep. 2025. PMID: 40547413 Free PMC article.
-
Neutrophil extracellular traps and interleukin-1β in cystic fibrosis lung disease.Front Immunol. 2025 Jul 28;16:1595994. doi: 10.3389/fimmu.2025.1595994. eCollection 2025. Front Immunol. 2025. PMID: 40791588 Free PMC article. Review.
-
Effect on consumers' sustainable purchase intention of dietary supplement purine labeling.Front Nutr. 2025 Jun 4;12:1526713. doi: 10.3389/fnut.2025.1526713. eCollection 2025. Front Nutr. 2025. PMID: 40535036 Free PMC article.
-
Local oxygen concentration reversal from hyperoxia to hypoxia monitored by optical-resolution photoacoustic microscopy in inflammation-resolution process.Photoacoustics. 2025 May 17;44:100730. doi: 10.1016/j.pacs.2025.100730. eCollection 2025 Aug. Photoacoustics. 2025. PMID: 40510562 Free PMC article.
-
Targeting Hyperuricemia and NLRP3 Inflammasome in Gouty Arthritis: A Preclinical Evaluation of Allopurinol and Disulfiram Combination Therapy.Pharmaceuticals (Basel). 2025 May 21;18(5):762. doi: 10.3390/ph18050762. Pharmaceuticals (Basel). 2025. PMID: 40430581 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
