Outcomes of Ahmed Glaucoma Valve Implantation with Subsequent Trans-Scleral Diode Cyclophotocoagulation as the Main Intervention if IOP Remained Medically Uncontrolled
- PMID: 39712369
- PMCID: PMC11662913
- DOI: 10.2147/OPTH.S498973
Outcomes of Ahmed Glaucoma Valve Implantation with Subsequent Trans-Scleral Diode Cyclophotocoagulation as the Main Intervention if IOP Remained Medically Uncontrolled
Abstract
Purpose: To evaluate the efficacy and safety of Ahmed glaucoma valve (AGV) implantation with subsequent trans-scleral diode cyclophotocoagulation (CPC) as the main intervention if IOP remained medically uncontrolled.
Patients and methods: Charts of 108 consecutive eyes (90 patients) that underwent AGV implantation from 2003 to 2018 at a single clinical practice were retrospectively reviewed. The procedure was considered a failure if any of the following occurred: additional incisional glaucoma surgery, IOP >21 mmHg or < 20% reduction from baseline on 2 consecutive study visits after 3 months, IOP ≤ 5 mmHg on 2 consecutive study visits after 3 months, loss of light perception, or AGV removal.
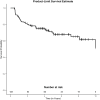
Results: The mean follow-up time was 5.4 ± 3.1 years. Diode CPC was performed in 32%. The mean IOP was 24.8 ± 8.2 mmHg before intervention, and 12.5 ± 5.6 mmHg at last follow-up (p<0.0001). The mean logMAR VA decreased by 0.24 (p=0.002). The success rate was 68%. The reasons for failure were additional incisional glaucoma surgery in 7%, AGV removal in 4%, loss of light perception in 4%, inadequate IOP reduction in 13%, and IOP ≤ 5 mm HG in 6%. The probability of survival by Kaplan Meier analysis was 88%, 76% and 69% at 1, 3, and 5 years after the procedure, respectively. Complications of AGV and CPC were comparable to those previously reported in the literature.
Conclusion: The treatment approach of AGV implantation with subsequent trans-scleral diode CPC, as needed, was successful in over 2/3rd of subjects. This study adds to the literature supporting the use of CPC when IOP is medically uncontrolled after AGV.
Keywords: cyclodestruction; laser; surgery; valved glaucoma implant.
© 2024 Radhakrishnan et al.
Conflict of interest statement
SR: Netra Systems Inc. (Consultant); AI: Belkin Lasers, Alcon, Ophthalmic Mutual Insurance Company (Consultant), and Eyenovia (Stock Owner). The authors report no other conflicts of interest this work.
Figures


Similar articles
-
Outcomes of Ahmed glaucoma valve and transscleral cyclophotocoagulation in neovascular glaucoma.Indian J Ophthalmol. 2022 Apr;70(4):1253-1259. doi: 10.4103/ijo.IJO_2107_21. Indian J Ophthalmol. 2022. PMID: 35326027 Free PMC article.
-
Transscleral cyclophotocoagulation with MicroPulse® laser versus Ahmed valve implantation in patients with advanced primary open-angle glaucoma.Int Ophthalmol. 2021 Apr;41(4):1271-1282. doi: 10.1007/s10792-020-01682-0. Epub 2021 Jan 3. Int Ophthalmol. 2021. PMID: 33392944 Clinical Trial.
-
Cyclophotocoagulation versus Ahmed Glaucoma Implant in Neovascular Glaucoma with Poor Vision at Presentation.Clin Ophthalmol. 2024 Jan 16;18:163-171. doi: 10.2147/OPTH.S424321. eCollection 2024. Clin Ophthalmol. 2024. PMID: 38250598 Free PMC article.
-
A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: a long-term follow-up.J Glaucoma. 2009 Mar;18(3):192-6. doi: 10.1097/IJG.0b013e31817d235c. J Glaucoma. 2009. PMID: 19295370
-
Cyclodestructive procedures for refractory glaucoma.Cochrane Database Syst Rev. 2019 Mar 10;3(3):CD012223. doi: 10.1002/14651858.CD012223.pub2. Cochrane Database Syst Rev. 2019. PMID: 30852841 Free PMC article.
References
LinkOut - more resources
Full Text Sources

