Frequency of ischemic cardiac events in patients receiving long-term multikinase inhibitor: A report of three cases
- PMID: 39712513
- PMCID: PMC11658567
- DOI: 10.1016/j.apjon.2024.100624
Frequency of ischemic cardiac events in patients receiving long-term multikinase inhibitor: A report of three cases
Abstract
Objective: To investigate the incidence and characteristics of ischemic cardiac events, specifically major adverse cardiac events (MACE), in patients undergoing long-term treatment with multikinase inhibitors (MKIs) such as lenvatinib and sorafenib.
Methods: A single-center retrospective analysis was conducted on 41 patients treated with lenvatinib or sorafenib for more than one year at our institution from 2015 to 2022. Patient records were reviewed to collect data on demographics, cancer type, cardiovascular risk factors, MKI treatment duration, and MACE incidence. MACE events, defined as acute heart failure, fatal arrhythmia, acute myocardial infarction, and coronary revascularization, were analyzed to determine potential correlations with MKI therapy.
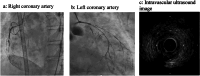
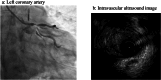
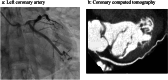
Results: Among the 41 patients, three (7.3%) developed MACE, presenting as acute heart failure, fatal arrhythmia, and acute myocardial infarction, all associated with significant coronary artery stenosis. Notably, none of these patients had a prior history of cardiovascular disease. Despite variations in clinical presentation, all cases suggested a link between long-term MKI administration and accelerated coronary atherosclerosis. Factors involved in atherosclerosis were significantly older and tended to be more hypertensive in the non-MACE group.
Conclusions: Long-term MKI therapy may increase the risk of severe ischemic cardiac events, likely due to accelerated atherosclerosis. Clinicians and oncology nurses should monitor patients closely for early signs of angina, especially in an outpatient setting, to prevent acute cardiac events. Further large-scale studies are warranted to establish a clearer causal relationship between MKI therapy and cardiovascular risks.
Keywords: Atherosclerosis; Cardio-oncology nursing; Cardiotoxicity management; Cardiovascular assessment; MACE; Multikinase inhibitor.
© 2024 The Author(s).
Conflict of interest statement
Dr. Kenmotsu received research funding from Ono Pharmaceutical Co, Ltd., Novartis Pharma K.K., Eli Lilly K.K, AstraZeneca K.K., Loxo Oncology, and speakers bureau from Amgen inc., AstraZeneca K.K., Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical Co, Ltd., Daiichi-Sankyo Co., Ltd., Eli Lilly K.K, Kyowa Hakko Kirin Co., Ltd., Merck, MSD, Novartis Pharma K.K., Ono Pharmaceutical Co, Ltd., Pfizer, Taiho Pharma, Takeda Pharmaceutical Co., Ltd.
Figures
References
LinkOut - more resources
Full Text Sources

