Weighing the Risk of Seizure Control: A Case of Levetiracetam-Induced Rhabdomyolysis
- PMID: 39712784
- PMCID: PMC11663018
- DOI: 10.7759/cureus.74111
Weighing the Risk of Seizure Control: A Case of Levetiracetam-Induced Rhabdomyolysis
Abstract
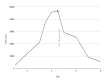
Rhabdomyolysis, a severe condition marked by the breakdown of muscle tissue, leads to the release of intracellular contents into the bloodstream. This condition can be triggered by a range of factors, including intense physical activity, traumatic injuries, certain medications, and infections. Diagnosis typically involves detecting elevated creatine phosphokinase (CPK) levels alongside characteristic clinical symptoms. Levetiracetam-induced rhabdomyolysis is an exceptionally rare phenomenon, with only a few cases documented in the literature. In this report, we present a 47-year-old male patient in our intensive care unit who developed rhabdomyolysis after continuing his home dose of levetiracetam following a witnessed seizure. Despite four days of aggressive hydration, his CPK levels continued to rise, ultimately peaking at 46,671 U/L. With no other apparent causes, levetiracetam was suspected as the culprit and subsequently discontinued. Remarkably, the patient's condition improved quickly after stopping the medication, with CPK levels dropping within two days, allowing for a successful transition to lacosamide. Although rare, this case highlights the critical need to monitor CPK levels in patients who develop rhabdomyolysis symptoms after restarting levetiracetam therapy. We recommend considering discontinuation of levetiracetam if no other identifiable causes are found.
Keywords: creatine kinase; levetiracetam; rhabdomyolysis; seizure; seizure medications.
Copyright © 2024, Lyles et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Newer antiepileptic drugs compared to levetiracetam as adjunctive treatments for uncontrolled focal epilepsy: an indirect comparison. Zhu LN, Chen D, Xu D, Tan G, Wang HJ, Liu L. Seizure. 2017;51:121–132. - PubMed
-
- Levetiracetam-induced rhabdomyolysis: analysis of reports from the Food and Drug Administration's Adverse Event Reporting System database. Carnovale C, Gentili M, Antoniazzi S, Clementi E, Radice S. Muscle Nerve. 2017;56:0–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources