Increasing Trends in the Pancreatitis Risk With Tumor Necrosis Factor Inhibitor Use
- PMID: 39712842
- PMCID: PMC11663436
- DOI: 10.7759/cureus.74245
Increasing Trends in the Pancreatitis Risk With Tumor Necrosis Factor Inhibitor Use
Abstract
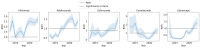
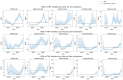
Tumor necrosis factor (TNF) inhibitors are a class of pharmacologic agents used to treat a wide range of immunologic diseases. We present a systematic study of the pancreatitis risk with TNF inhibitor use through a retrospective case-control design disproportionality analysis of the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) database, with data ranging from the fourth quarter of 2012 (2012 Q4) to the second quarter of 2024 (2024 Q2). Our analysis reveals an increasing trend in the pancreatitis risk with TNF inhibitors when stratified by year, with all of the TNF inhibitors studied exhibiting significant association with pancreatitis in the year 2023. Furthermore, these results hold even when controlling for sex, age, concurrent use of azathioprine, and the indication for treatment. Our findings flag the necessity for a careful investigation and monitoring of pancreatitis risk in patients undergoing TNF inhibitor therapy, especially in recent years.
Keywords: disproportionality analysis; food and drug administration adverse event reporting system (faers); medication-induced pancreatitis; pharmacovigilance; tnf alpha inhibitor.
Copyright © 2024, Gu et al.
Conflict of interest statement
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study. Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue. Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures

Similar articles
-
Pancreatitis Associated With Teduglutide: A Disproportionality Analysis via the Food and Drug Administration Adverse Event Reporting System (FAERS) Database.Cureus. 2024 Aug 29;16(8):e68091. doi: 10.7759/cureus.68091. eCollection 2024 Aug. Cureus. 2024. PMID: 39350843 Free PMC article.
-
Adverse event profiles of CDK4/6 inhibitors: data mining and disproportionality analysis of the FDA adverse event reporting system.Ther Adv Drug Saf. 2024 Sep 24;15:20420986241278498. doi: 10.1177/20420986241278498. eCollection 2024. Ther Adv Drug Saf. 2024. PMID: 39376495 Free PMC article.
-
Metformin adverse event profile: a pharmacovigilance study based on the FDA Adverse Event Reporting System (FAERS) from 2004 to 2022.Expert Rev Clin Pharmacol. 2024 Jan-Jun;17(2):189-201. doi: 10.1080/17512433.2024.2306223. Epub 2024 Jan 29. Expert Rev Clin Pharmacol. 2024. PMID: 38269492
-
SGLT2 inhibitors associated pancreatitis: signal identification through disproportionality analysis of spontaneous reports and review of case reports.Int J Clin Pharm. 2022 Dec;44(6):1425-1433. doi: 10.1007/s11096-022-01476-7. Epub 2022 Oct 12. Int J Clin Pharm. 2022. PMID: 36224513 Review.
-
Risk of pancreatitis and pancreatic carcinoma for anti-diabetic medications: findings from real-world safety data analysis and systematic review and meta-analysis of randomized controlled trials.Expert Opin Drug Saf. 2024 Jun;23(6):731-742. doi: 10.1080/14740338.2023.2284992. Epub 2023 Dec 11. Expert Opin Drug Saf. 2024. PMID: 37986140
References
-
- Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Pharmacol Ther. 2008;117:244–279. - PubMed
LinkOut - more resources
Full Text Sources
