Impact of Chronic Intermittent Hypoxia on Cognitive Function and Hippocampal Neurons in Mice: A Study of Inflammatory and Oxidative Stress Pathways
- PMID: 39712883
- PMCID: PMC11660659
- DOI: 10.2147/NSS.S489232
Impact of Chronic Intermittent Hypoxia on Cognitive Function and Hippocampal Neurons in Mice: A Study of Inflammatory and Oxidative Stress Pathways
Abstract
Purpose: Chronic intermittent hypoxia (CIH) is considered one of the main pathophysiological mechanisms of obstructive sleep apnea (OSA). CIH can further lead to cognitive dysfunction by inducing processes such as neuroinflammation and oxidative stress. The hippocampus is primarily associated with cognitive functions such as learning and memory. This study aimed to explore the effects of CIH on cognitive function and hippocampal neurons in mice and to reveal its potential molecular mechanisms.
Methods: SPF-grade C57BL/6J mice (n=36) were selected as subjects and divided into control, mild CIH, and severe CIH groups (12 mice per group). Cognitive function was assessed using the Morris water maze test, and hippocampal neuron numbers and morphological changes were observed using HE staining and Nissl staining. Additionally, differential genes and pathways were revealed through RNA sequencing (RNA-seq) and bioinformatics analysis. We examined oxidative stress-related biochemical markers in the hippocampal tissue and used Western Blot to verify changes in the expression of potential key genes. Statistical analyses were performed using ANOVA and post hoc tests to ensure robust comparisons between groups.
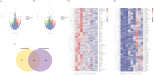
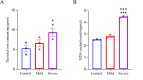
Results: CIH mice exhibited significant cognitive impairment, including decreased learning and memory abilities. The severe CIH group had a longer escape latency compared to the mild CIH group (p < 0.001) and the control group (p < 0.01), while the mild CIH group took longer than the control group (p < 0.01). In the probe test, the severe CIH group showed a significant decrease in platform crossings (p < 0.01) and target quadrant dwell time (p < 0.05), while the mild CIH group exhibited a reduction in target quadrant dwell time (p < 0.05). Abnormal hippocampal neuron morphology was observed, with a significant reduction in hippocampal neurons (p < 0.05). RNA-seq analysis revealed numerous differentially expressed genes, mainly enriched in biological processes such as inflammation and oxidative stress, as well as multiple signaling pathways. Specifically, downregulated LepR, SIRT1, and Nrf2 genes were found to exacerbate oxidative stress and neuroinflammation, impairing neuronal integrity and cognitive function. Further validation showed increased oxidative stress levels in hippocampal tissue and downregulation of key gene expression. Western blot analysis confirmed significantly reduced expression of LepR (p < 0.01), SIRT1 (p < 0.001), and Nrf2 (p < 0.001) in the severe CIH group.
Conclusion: While oxidative stress and inflammation are well-established mechanisms in CIH-induced cognitive impairment, our study provides novel insights by identifying the specific roles of LepR, SIRT1, and Nrf2 in this process. The downregulation of these key genes suggests potential new targets for therapeutic intervention. Importantly, the differential expression patterns observed in varying degrees of hypoxia severity highlight the potential for tailored therapeutic strategies that modulate these pathways in response to the intensity of hypoxic exposure. These findings offer unique opportunities for developing targeted therapies aimed at mitigating CIH-related cognitive decline and neural damage. However, a key limitation of this study is the exclusive use of animal models, which may not fully replicate human pathophysiology. Further studies are needed to validate these findings in clinical settings and to explore the regulatory relationships between the key genes involved.
Keywords: RNA sequencing; chronic intermittent hypoxia; cognitive impairment; hippocampal neurons; inflammation; obstructive sleep apnea; oxidative stress.
© 2024 Zhang et al.
Conflict of interest statement
The authors declare no conflicts of interest related to this study.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

