Salt stress affects the bacterial communities in rhizosphere soil of rice
- PMID: 39712891
- PMCID: PMC11659233
- DOI: 10.3389/fmicb.2024.1505368
Salt stress affects the bacterial communities in rhizosphere soil of rice
Abstract
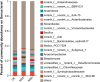
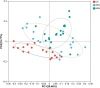
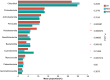
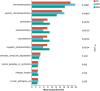
Salt is a primary factor limiting the utilization of saline lands in coastal beach areas, with rhizosphere microorganisms playing a crucial role in enhancing crop stress resistance and exhibiting high sensitivity to environmental changes. Rice (Oryza sativa L.) is the preferred crop for reclaiming salinized soils. This study determined the microbial communities in rhizosphere soil of rice under different salt stress treatments by high-throughput sequencing. We found that salt stress changed the bacterial community diversity, structure and function in rhizosphere soil of rice. Salt stress significantly reduced the richness and diversity of bacterial communities in rhizosphere soil of rice. The bacterial community was characterized by higher abundance of the phyla Chloroflexi, Proteobacteria and Actinobacteria; the relative abundances of Firmicutes, Acidobacteriota and Myxococcota were decreased, while Bacteroidota and Cyanobacteria were increased under salt stress. The functions of bacterial communities in rhizosphere soil of rice mainly include chemoheterotrophy, aerobic_chemoheterotrophy, phototrophy etc., chemoheterotrophy and aerobic_chemoheterotrophy were significantly higher NS3 (adding 3‰ NaCl solution to the base soil) treatment than NS6 (adding 6‰ NaCl solution to the base soil) treatment. These findings provide a theoretical foundation for the development of specialized salt-tolerant microbial agents for rice cultivation and offer a viable strategy for improving the soil environment of saline coastal lands through the application of beneficial microorganisms.
Keywords: 16S rRNA; Oryza sativa L.; bacterial community; rhizosphere soil; salt stress.
Copyright © 2024 Zhou, He, Lin, Lin, Long, Xie and Hu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Rice SST Variation Shapes the Rhizosphere Bacterial Community, Conferring Tolerance to Salt Stress through Regulating Soil Metabolites.mSystems. 2020 Nov 24;5(6):e00721-20. doi: 10.1128/mSystems.00721-20. mSystems. 2020. PMID: 33234605 Free PMC article.
-
[Effects of Aeration Methods on Microbial Diversity and Community Structure in Rice Rhizosphere].Huan Jing Ke Xue. 2023 Nov 8;44(11):6362-6376. doi: 10.13227/j.hjkx.202211311. Huan Jing Ke Xue. 2023. PMID: 37973118 Chinese.
-
[Effects of Selected Biochars Application on the Microbial Community Structures and Diversities in the Rhizosphere of Water Spinach (Ipomoea aquatica Forssk.) Irrigated with Reclaimed Water].Huan Jing Ke Xue. 2020 Dec 8;41(12):5636-5647. doi: 10.13227/j.hjkx.202006087. Huan Jing Ke Xue. 2020. PMID: 33374081 Chinese.
-
Rhizosphere bacteria community and functions under typical natural halophyte communities in North China salinized areas.PLoS One. 2021 Nov 11;16(11):e0259515. doi: 10.1371/journal.pone.0259515. eCollection 2021. PLoS One. 2021. PMID: 34762689 Free PMC article.
-
Influence of salt stress on the rhizosphere soil bacterial community structure and growth performance of groundnut (Arachis hypogaea L.).Int Microbiol. 2020 Aug;23(3):453-465. doi: 10.1007/s10123-020-00118-0. Epub 2020 Jan 13. Int Microbiol. 2020. PMID: 31933013
Cited by
-
Proteus sp. Strain JHY1 Synergizes with Exogenous Dopamine to Enhance Rice Growth Performance Under Salt Stress.Microorganisms. 2025 Aug 4;13(8):1820. doi: 10.3390/microorganisms13081820. Microorganisms. 2025. PMID: 40871325 Free PMC article.
-
Cooperative Interplay Between PGPR and Trichoderma longibrachiatum Reprograms the Rhizosphere Microecology for Improved Saline Alkaline Stress Resilience in Rice Seedlings.Microorganisms. 2025 Jul 2;13(7):1562. doi: 10.3390/microorganisms13071562. Microorganisms. 2025. PMID: 40732070 Free PMC article.
-
Enhancement of environmental microplastics (MPs) degradation via bacteria under stress conditions: key enzymes, pathways, and mechanisms.World J Microbiol Biotechnol. 2025 Aug 26;41(9):318. doi: 10.1007/s11274-025-04525-1. World J Microbiol Biotechnol. 2025. PMID: 40856897 Review.
-
Soil Bacterial Community Characteristics and Functional Analysis of Estuarine Wetlands and Nearshore Estuarine Wetlands in Qinghai Lake.Microorganisms. 2025 Mar 27;13(4):759. doi: 10.3390/microorganisms13040759. Microorganisms. 2025. PMID: 40284596 Free PMC article.
-
Effects of Potassium Fulvic Acid on Soil Physical and Chemical Properties and Soil Microenvironment of Blueberry (Vaccinium corymbosum L.) Under Salt Stress.Plants (Basel). 2025 May 29;14(11):1654. doi: 10.3390/plants14111654. Plants (Basel). 2025. PMID: 40508328 Free PMC article.
References
-
- Baumann K., Dignac M.-F., Rumpel C., Bardoux G., Sarr A., Steffens M., et al. . (2013). Soil microbial diversity affects soil organic matter decomposition in a silty grassland soil. Biogeochemistry 114, 201–212. doi: 10.1007/s10533-012-9800-6 - DOI
-
- Bello A. S., Ben-Hamadou R., Hamdi H., Saadaoui I., Ahmed T. (2021). Application of cyanobacteria (Roholtiella sp.) liquid extract for the alleviation of salt stress in bell pepper (capsicum annuum L.) plants grown in a soilless system. Plan. Theory 11:104. doi: 10.3390/plants11010104, PMID: - DOI - PMC - PubMed
-
- Bryant D. A., Liu Z., Li T., Zhao F., Costas A. M. G., Klatt C. G., et al. . (2012). Comparative and functional genomics of anoxygenic green bacteria from the taxa Chlorobi, Chloroflexi, and Acidobacteria. Funct. Genomics Evol. Photosynthetic Syst. 22, 47–102. doi: 10.1007/978-94-007-1533-2_3 - DOI
LinkOut - more resources
Full Text Sources

