The Effects of Perfluorooctanesulfonic acid (PFOS) on Human Umbilical Vein Endothelial Cells (HUVECs) Proliferation and Gene Expression and its Implications on Fetal Development
- PMID: 39712934
- PMCID: PMC11659881
- DOI: 10.17912/micropub.biology.001318
The Effects of Perfluorooctanesulfonic acid (PFOS) on Human Umbilical Vein Endothelial Cells (HUVECs) Proliferation and Gene Expression and its Implications on Fetal Development
Abstract
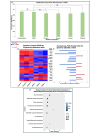
Polyfluoro-alkyl substances (PFAS) are widely distributed environmental contaminants linked to human toxicity and developmental delays, especially low birthweight (LBW). In this study, Human Umbilical Vein Endothelial Cells (HUVECs) were exposed to the PFAS perfluorooctanesulfonic acid (PFOS). After 48-hours, their proliferation, and differential gene expression were assessed. A small, yet significant, reduction in proliferation was seen at 50 μg/mL and 75 μg/mL. RNA sequencing showed that estrogen response and notch signaling pathways were significantly altered. This study increases our understanding of how PFAS may interfere with endothelial cell (HUVECs) functions which may have larger effects on fetal growth, development, and birthweight.
Copyright: © 2024 by the authors.
Conflict of interest statement
The authors declare that there are no conflicts of interest present.
Figures
References
-
- Akil Abdellah, Gutiérrez-García Ana K., Guenter Rachael, Rose J. Bart, Beck Adam W., Chen Herbert, Ren Bin. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Frontiers in Cell and Developmental Biology. 2021 Feb 16;9 doi: 10.3389/fcell.2021.642352. - DOI - PMC - PubMed
-
- Behr Anne-Cathrin, Lichtenstein Dajana, Braeuning Albert, Lampen Alfonso, Buhrke Thorsten. Perfluoroalkylated substances (PFAS) affect neither estrogen and androgen receptor activity nor steroidogenesis in human cells in vitro. Toxicology Letters. 2018 Jul 1;291:51–60. doi: 10.1016/j.toxlet.2018.03.029. - DOI - PubMed
-
- Bonato Marco, Corrà Francesca, Bellio Marta, Guidolin Laura, Tallandini Laura, Irato Paola, Santovito Gianfranco. PFAS Environmental Pollution and Antioxidant Responses: An Overview of the Impact on Human Field. International Journal of Environmental Research and Public Health. 2020 Oct 30;17(21):8020–8020. doi: 10.3390/ijerph17218020. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
