Phosphopeptide Neoantigens as Emerging Targets in Cancer Immunotherapy
- PMID: 39713021
- PMCID: PMC11661817
- DOI: 10.33696/cancerimmunol.6.094
Phosphopeptide Neoantigens as Emerging Targets in Cancer Immunotherapy
Abstract
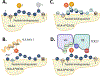
Protein post-translational modifications play a vital role in various cellular events essential for maintaining cellular physiology and homeostasis. In cancer cells, aberrant post-translational modifications such as glycosylation, acetylation, and phosphorylation on proteins can result in the generation of antigenic peptide variants presented in complex with MHC molecules. These modified peptides add to the class of tumorspecific antigens and offer promising avenues for targeted anti- cancer therapies. In this review, we focus on the role of phosphorylated peptides (p-peptides) in cancer immunity. We discuss the mechanisms by which the phosphorylated moiety modifies the structural features and binding properties of p-peptides with MHC, compared to their non-phosphorylated counterparts. Additionally, we review recent work on how the HLA-B*07-specific p-peptide, pMLL747-755, interacts with its cognate TCR. Altogether, p-peptides are emerging as a novel class of tumor-specific antigens, expanding the range of targets in cancer immunotherapy.
Keywords: Immune checkpoint blockade therapies; Immunopeptidome; Neoantigens; Phosphorylation; Post-translational modifications.
Conflict of interest statement
MK previously received support from Agenus for published work on phosphoantigens described here. MK serves as an advisor for Merck Sharp and Dohme. MK currently receives research support from Merck Sharp and Dohme, Genentech, Biogen and Novartis which is not related to this work.
Figures

References
-
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015. Jun 25;372(26):2521–32. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
