Design and Optimization of Isothermal Gene Amplification for Generation of High-Gain Oligonucleotide Products by MicroRNAs
- PMID: 39713023
- PMCID: PMC11660000
- DOI: 10.1021/acsmeasuresciau.4c00063
Design and Optimization of Isothermal Gene Amplification for Generation of High-Gain Oligonucleotide Products by MicroRNAs
Abstract
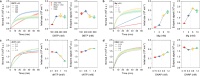
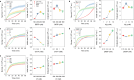
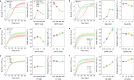
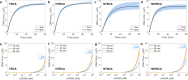
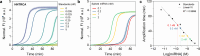
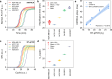
Thermal cycling-based quantitative polymerase chain reaction (qPCR) represents the gold standard method for accurate and sensitive nucleic acid quantification in laboratory settings. However, its reliance on costly thermal cyclers limits the implementation of this technique for rapid point-of-care (POC) diagnostics. To address this, isothermal amplification techniques such as rolling circle amplification (RCA) have been developed, offering a simpler alternative that can operate without the need for sophisticated instrumentation. This study focuses on the development and optimization of toehold-mediated RCA (TRCA), which employs a conformationally switchable dumbbell DNA template for the sensitive and selective detection of cancer-associated miRNAs, specifically miR-21. In addition, we developed variants of hyperbranched TRCA (HTRCA), nicking-assisted TRCA (NTRCA), and hyperbranched NTRCA (HNTRCA) to facilitate exponential amplification by enhancing TRCA through the sequential incorporation of reverse primer (Pr) and nicking endonuclease (nE). By conducting a systematic kinetic analysis of the initial rate and end point signals for varying concentrations of key reaction components, we could identify optimal conditions that markedly enhanced the sensitivity and specificity of the TRCA variants. In particular, HNTRCA, which exploits the synergistic effect of Pr and nE, demonstrated an approximately 3000-fold improvement in the detection limit (260 fM) and a wider dynamic range of more than 4 log orders of magnitude compared to TRCA, thereby evidencing its superior performance. Also, we established a mechanistic model for TRCA that includes the roles of Pr and nE in different amplification processes. Model parameters were fitted to the experimental data, and additional simulations were conducted to compare the four amplification methods. Further tests with real biological samples revealed that this technique showed a good correlation with qPCR in quantifying miR-21 expression in various cell lines (0.9510 of Pearson's r), confirming its potential as a robust and rapid tool for nucleic acid detection. Therefore, the simplicity, high sensitivity, and potential for integration with POC diagnostic platforms make the HNTRCA system suitable for field deployment in resource-limited environments.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Sensitive fluorescent detection of DNA methyltransferase using nicking endonuclease-mediated multiple primers-like rolling circle amplification.Biosens Bioelectron. 2017 May 15;91:417-423. doi: 10.1016/j.bios.2016.12.061. Epub 2016 Dec 30. Biosens Bioelectron. 2017. PMID: 28063390
-
Rapid quantification of microRNA-375 through one-pot primer-generating rolling circle amplification.Analyst. 2022 Jun 27;147(13):2936-2941. doi: 10.1039/d2an00263a. Analyst. 2022. PMID: 35695478 Free PMC article.
-
One-step isothermal amplification strategy for microRNA specific and ultrasensitive detection based on nicking-assisted entropy-driven DNA circuit triggered exponential amplification reaction.Anal Chim Acta. 2022 Apr 22;1203:339706. doi: 10.1016/j.aca.2022.339706. Epub 2022 Mar 10. Anal Chim Acta. 2022. PMID: 35361424
-
Rolling Circle Amplification in Integrated Microsystems: An Uncut Gem toward Massively Multiplexed Pathogen Diagnostics and Genotyping.Acc Chem Res. 2021 Nov 2;54(21):3979-3990. doi: 10.1021/acs.accounts.1c00438. Epub 2021 Oct 12. Acc Chem Res. 2021. PMID: 34637281 Free PMC article. Review.
-
Strategies for Engineering Affordable Technologies for Point-of-Care Diagnostics of Infectious Diseases.Acc Chem Res. 2021 Oct 19;54(20):3772-3779. doi: 10.1021/acs.accounts.1c00434. Epub 2021 Oct 6. Acc Chem Res. 2021. PMID: 34612619 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
