Phase II trial of blood-brain barrier permeable peptide-paclitaxel conjugate ANG1005 in patients with recurrent high-grade glioma
- PMID: 39713041
- PMCID: PMC11662161
- DOI: 10.1093/noajnl/vdae186
Phase II trial of blood-brain barrier permeable peptide-paclitaxel conjugate ANG1005 in patients with recurrent high-grade glioma
Abstract
Background: This study is a phase II clinical trial to evaluate the efficacy, safety, and tolerability of the blood-brain barrier (BBB) permeable peptide-paclitaxel conjugate ANG1005 in patients with recurrent high-grade glioma (HGG) (NCT01967810).
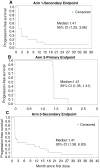
Methods: Seventy-three patients were enrolled in 3 separate arms-recurrent glioblastoma (GBM) (Arm 1), bevacizumab refractory GBM (Arm 2), and grade 3 anaplastic gliomas (AGs) (Arm 3). The study was started in October 2013, and the data were locked on September 29, 2017. Safety was evaluated for all three arms (n = 73), and the primary endpoint for Arms 1 and 3 was objective response rate (ORR), and Arm 2 primary endpoint was progression-free survival rate at 3 months (PFS3).
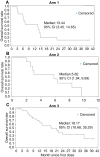
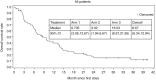
Results: Overall, the safety of ANG1005 was found to be consistent with a taxane toxicity profile. Otherwise, the primary efficacy endpoints of ORR and PFS were not met. The most common adverse events (AEs) were hematologic (32.9%), alopecia (31.5%), and fatigue (30.1%). The median PFS was 1.4 months (95% CI: 1.4, 2.1) and similar across all the treatment arms. The median overall survival was 13.4 months (95% CI: 3.4, 14.6) in Arm 1, 5.8 months (95% CI: 1.9, 9.7) in Arm 2, and 18.2 months (95% CI: 10.7, 35.3) in Arm 3.
Conclusion: A dose of 600 mg/m2 was determined to be safe in this study. However, the primary efficacy endpoint was not met in the NCT01967810-ANG1005 trial, and no further studies are planned in the glioma setting with this compound.
Keywords: ANG1005; high-grade glioma; paclitaxel; phase II clinical trial.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
P.K. holds ownership interest (including patents) in Angiochem and is an advisory board member/unpaid consultant for Elevate Bio. A.M.S. is a consultant for Carthera and Angiochem. A.M.S. has received in-kind and funding support from Carthera, Agenus, and BMS. J.D. receives research support from Novartis, Servier, Novocure, hemerion is a consultant with Servier, Elsevier, and has stocks with Gilead, Pfizer, GSK, Wolters Kluwer. M.A. is a consultant for Bayer, Novocure, Kiyatec, Insightec, GSK, Xoft, Nuvation, Cellularity, SDP Oncology, Apollomics, Prelude, Janssen, Tocagen, Voyager Therapeutics, Viewray, Caris Lifesciences, Pyramid Biosciences, Varian Medical Systems, Cairn Therapeutics, Anheart Therapeutics, Theraguix, Menarini Ricerche, Sumitomo Pharma Oncology, Autem therapeutics, GT Medical Technologies, Allovir. He is on the scientific advisory board of Cairn Therapeutics, Modifi biosciences. Bugworks and holds stock options with Mimivax, Cytodyn, MedInnovate Advisors LLC, Trisalus Lifesciences. All the other authors declare that they have no competing interests.
Figures
References
-
- Motl S, Zhuang Y, Waters CM, et al. Pharmacokinetic considerations in the treatment of CNS tumours. Clin Pharmacokinet. 2006; 45(9):871–903. - PubMed
-
- Wen PY, Kesari S.. Malignant gliomas in adults. N Engl J Med. 2008; 359(5):492–507. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352(10):987–996. - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
