Collagen scaffold-seeded iTenocytes accelerate the healing and functional recovery of Achilles tendon defects in a rat model
- PMID: 39713100
- PMCID: PMC11658981
- DOI: 10.3389/fbioe.2024.1407729
Collagen scaffold-seeded iTenocytes accelerate the healing and functional recovery of Achilles tendon defects in a rat model
Abstract
Introduction: Tendon injuries represent an ongoing challenge in clinical practice due to poor regenerative capacity, structure, and biomechanical function recovery of ruptured tendons. This study is focused on the assessment of a novel strategy to repair ruptured Achilles tendons in a Nude rat model using stem cell-seeded biomaterial.
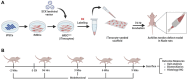
Methods: Specifically, we have used induced pluripotent stem cell (iPSC)-derived mesenchymal stem cells (iMSCs) overexpressing the early tendon marker Scleraxis (SCX, iMSCSCX+, iTenocytes) in combination with an elastic collagen scaffold. Achilles tendon defects in Nude rat models were created by isolating the tendon and excising 3 mm of the midsection. The Achilles tendon defects were then repaired with iTenocyte-seeded scaffolds, unseeded scaffolds, or suture only and compared to native Nude rat tendon tissue using gait analyses, biomechanical testing, histology, and immunohistochemistry.
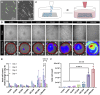
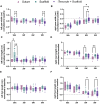
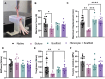
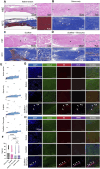
Results: The results show faster functional recovery of gait in iTenocyte-seeded scaffold group comparing to scaffold only and suture only groups. Both iTenocyte-seeded scaffold and scaffold only treatment groups had improved biomechanical properties when compared to suture only treatment group, however no statistically significant difference was found in comparing the cell seeding scaffold an scaffold only group in terms of biomechanical properties. Immunohistochemistry staining further demonstrated that iTenocytes successfully populated the collagen scaffolds and survived 9 weeks after implantation in vivo. Additionally, the repaired tissue of iTenocyte-treated injuries exhibited a more organized structure when compared to tendon defects that were repaired only with suturing or unseeded scaffolds.
Conclusion: We suggest that iTenocyte-seeded DuRepair™ collagen scaffold can be used as potential treatment to regenerate the tendon tissue biomechanically and functionally.
Keywords: Achilles tendon rupture repair; collagen scaffold; stem cells; tissue engineering; tissue regeneration.
Copyright © 2024 Später, Del Rio, Shelest, Wechsler, Kaneda, Chavez, Sheyn, Yu, Metzger, Huang, Metzger, Tawackoli and Sheyn.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Brown J. P., Galassi T. V., Stoppato M., Schiele N. R., Kuo C. K. (2015). Comparative analysis of mesenchymal stem cell and embryonic tendon progenitor cell response to embryonic tendon biochemical and mechanical factors. Stem Cell Res. Ther. 6 (1), 89–98. 10.1186/s13287-015-0043-z - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

