Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy
- PMID: 39713208
- PMCID: PMC11655317
- DOI: 10.1002/EXP.20230171
Tumor cell membrane-based vaccines: A potential boost for cancer immunotherapy
Abstract
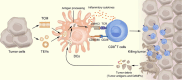
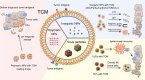
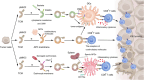
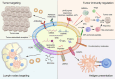
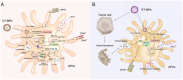
Because therapeutic cancer vaccines can, in theory, eliminate tumor cells specifically with relatively low toxicity, they have long been considered for application in repressing cancer progression. Traditional cancer vaccines containing a single or a few discrete tumor epitopes have failed in the clinic, possibly due to challenges in epitope selection, target downregulation, cancer cell heterogeneity, tumor microenvironment immunosuppression, or a lack of vaccine immunogenicity. Whole cancer cell or cancer membrane vaccines, which provide a rich source of antigens, are emerging as viable alternatives. Autologous and allogenic cellular cancer vaccines have been evaluated as clinical treatments. Tumor cell membranes (TCMs) are an intriguing antigen source, as they provide membrane-accessible targets and, at the same time, serve as integrated carriers of vaccine adjuvants and other therapeutic agents. This review provides a summary of the properties and technologies for TCM cancer vaccines. Characteristics, categories, mechanisms, and preparation methods are discussed, as are the demonstrable additional benefits derived from combining TCM vaccines with chemotherapy, sonodynamic therapy, phototherapy, and oncolytic viruses. Further research in chemistry, biomedicine, cancer immunology, and bioinformatics to address current drawbacks could facilitate the clinical adoption of TCM vaccines.
Keywords: cancer immunotherapy; cancer vaccine; tumor cell membrane; tumor‐derived extracellular vesicles.
© 2024 The Author(s). Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
