Pharmacokinetics/pharmacodynamics of glucocorticoids: modeling the glucocorticoid receptor dynamics and dose/response of commonly prescribed glucocorticoids
- PMID: 39713253
- PMCID: PMC11661806
- DOI: 10.5599/admet.2414
Pharmacokinetics/pharmacodynamics of glucocorticoids: modeling the glucocorticoid receptor dynamics and dose/response of commonly prescribed glucocorticoids
Abstract
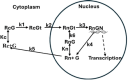
Background and purpose: The main features of the dynamics of the glucocorticoid receptor (GR) have been known for 50 years: 1) in the absence of glucocorticoid (G), the receptor is localized entirely in the cytoplasm; 2) upon G binding, GR is converted into a tightly bound G form and is rapidly imported into the nucleus where it can bind DNA and modulate transcription; 3) nuclear export of GR is very slow; and 4) the nuclear form of GR can recycle through an unbound form, back to the bound transcription modulating form without leaving the nucleus.
Experimental approach: A kinetic model that captures these features is presented, a set of model parameters for dexamethasone is derived, and the clinical implication for the commonly used glucocorticoids is discussed.
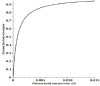
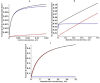
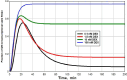
Key results: At the high concentrations normally used to describe G pharmacodynamics, the model reduces to the standard Michaelis-Menten equation with a K m that is a function of 4 model parameters. At very low concentrations, it reduces to another Michaelis-Menten equation with about a 1000-fold greater affinity, eg. at the nadir human endogenous cortisol concentration, the full model GR activity is 2.6 times greater than that predicted by extrapolation of the high concentration results.
Conclusion: The model is used to relate normal human 24-hour endogenous plasma cortisol levels to transcriptional activity and is applied to the commonly prescribed glucocorticoids (dexamethasone, methylprednisolone, prednisone) in an attempt to provide a pharmacological rationale for the very large therapeutic dosage range that has been traditionally used.
Keywords: Dexamethasone; cortisol; methylprednisolone; prednisolone; prednisone; transcription.
Copyright © 2024 by the authors.
Conflict of interest statement
Conflicts of Interest: The author certifies that he has no financial or other conflict of interest.
Figures

Similar articles
-
Glucocorticoid receptor-independent transcriptional induction of cytochrome P450 3A1 by metyrapone and its potentiation by glucocorticoid.Mol Pharmacol. 1996 Oct;50(4):856-63. Mol Pharmacol. 1996. PMID: 8863830
-
First High-Resolution Crystal Structures of the Glucocorticoid Receptor Ligand-Binding Domain-Peroxisome Proliferator-Activated γ Coactivator 1-α Complex with Endogenous and Synthetic Glucocorticoids.Mol Pharmacol. 2019 Oct;96(4):408-417. doi: 10.1124/mol.119.116806. Epub 2019 Aug 7. Mol Pharmacol. 2019. PMID: 31391291 Free PMC article.
-
Differences in down-regulation of glucocorticoid receptor mRNA by cortisol, prednisolone and dexamethasone in HeLa cells.Endocr J. 1995 Oct;42(5):629-36. doi: 10.1507/endocrj.42.629. Endocr J. 1995. PMID: 8574285
-
Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids.Clin Pharmacokinet. 2005;44(1):61-98. doi: 10.2165/00003088-200544010-00003. Clin Pharmacokinet. 2005. PMID: 15634032 Review.
-
On the trail of the glucocorticoid receptor: into the nucleus and back.Traffic. 2012 Mar;13(3):364-74. doi: 10.1111/j.1600-0854.2011.01288.x. Epub 2011 Oct 17. Traffic. 2012. PMID: 21951602 Review.
Cited by
-
Glucocorticoid Insensitivity: Is It a Question of Time and Place?Biomedicines. 2025 Jun 10;13(6):1418. doi: 10.3390/biomedicines13061418. Biomedicines. 2025. PMID: 40564137 Free PMC article. Review.
References
-
- Munck A., Brinck-Johnsen T.. Specific and nonspecific physicochemical interactions of glucocorticoids and related steroids with rat thymus cells in vitro. J Biol Chem 243 (1968) 5556-5565. https://www.ncbi.nlm.nih.gov/pubmed/5699052. - PubMed
-
- Munck A.. Glucocorticoid receptors and physiology: a personal history. Steroids 70 (2005) 335-344.https://doi.org/10.1016/j.steroids.2004.12.002. 10.1016/j.steroids.2004.12.002 - DOI - PubMed
-
- Munck A., Wira C., Young D.A., Mosher K.M., Hallahan C., Bell P.A.. Glucocorticoid-receptor complexes and the earliest steps in the action of glucocorticoids on thymus cells. J Steroid Biochem 3 (1972) 567-578.https://doi.org/10.1016/0022-4731(72)90103-3. 10.1016/0022-4731(72)90103-3 - DOI - PubMed
-
- Orti E., Mendel D.B., Smith L.I., Bodwell J.E., Munck A.. A dynamic model of glucocorticoid receptor phosphorylation and cycling in intact cells. J Steroid Biochem 34 (1989) 85-96.https://doi.org/10.1016/0022-4731(89)90069-1. 10.1016/0022-4731(89)90069-1 - DOI - PubMed
-
- Bledsoe R.K., Montana V.G., Stanley T.B., Delves C.J., Apolito C.J., McKee D.D., Consler T.G., Parks D.J., Stewart E.L., Willson T.M., Lambert M.H., Moore J.T., Pearce K.H., Xu H.E.. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 110 (2002) 93-105.https://doi.org/10.1016/s0092-8674(02)00817-6. 10.1016/s0092-8674(02)00817-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
