Construction of a CYP2J2-Template System and Its Application for Ligand Metabolism Prediction
- PMID: 39713276
- PMCID: PMC11649976
- DOI: 10.14252/foodsafetyfscj.D-24-00010
Construction of a CYP2J2-Template System and Its Application for Ligand Metabolism Prediction
Abstract
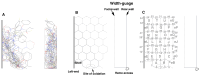
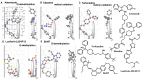
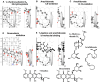
A Template system for the understanding of human CYP2J2-mediated reactions was constructed from the assembly of the ligands with the introduction of ideas of allowable width, Trigger-residue and the residue-initiated movement of ligands in the active site, which were in common with other Template* systems for human CYP1A1, CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2E1, CYP3A4, CYP3A5, and CYP3A7 (Drug Metab Pharmacokinet 2016, 2017, 2019, 2020, 2021, 2022, 2023, 2024, and in press 2024). CYP2J2 system also includes ideas of bi-molecule binding of ligands on the Template. From their placements on the Template and rules for interaction modes, verifications of good and poor substrates, regio/stereo-selectivity, and inhibitory interaction became available faithfully for these ligands. The refined CYP2J2-Template system will thus offer reliable estimations of this human CYP catalysis toward ligands of diverse structures, together with their deciphering information to lead to judgments.
Keywords: CYP2J2-mediated metabolism; fused-grid Template; ligand immobilization; poor and good substrates; simulation of ligand-interaction on Template..
©2024 Food Safety Commission, Cabinet Office, Government of Japan.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures