This is a preprint.
Top-down attention and Alzheimer's pathology impact cortical selectivity during learning, influencing episodic memory in older adults
- PMID: 39713293
- PMCID: PMC11661099
- DOI: 10.1101/2024.12.04.626911
Top-down attention and Alzheimer's pathology impact cortical selectivity during learning, influencing episodic memory in older adults
Update in
-
Top-down attention and Alzheimer's pathology affect cortical selectivity during learning, influencing episodic memory in older adults.Sci Adv. 2025 Jun 13;11(24):eads4206. doi: 10.1126/sciadv.ads4206. Epub 2025 Jun 13. Sci Adv. 2025. PMID: 40512843 Free PMC article.
Abstract
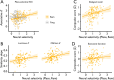
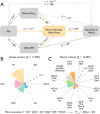
Human aging affects the ability to remember new experiences, in part, because of altered neural function during memory formation. One potential contributor to age-related memory decline is diminished neural selectivity -- i.e., a decline in the differential response of cortical regions to preferred vs. non-preferred stimuli during event perception -- yet the factors driving variability in neural selectivity with age remain unclear. We examined the impact of top-down attention and preclinical Alzheimer's disease (AD) pathology on neural selectivity during memory encoding in 156 cognitively unimpaired older participants who underwent fMRI while performing a word-face and word-scene associative memory task. Neural selectivity in face- and place-selective cortical regions was greater during events that were later remembered compared to forgotten. Critically, neural selectivity during learning positively scaled with memory-related variability in top-down attention, whereas selectivity negatively related to early AD pathology, evidenced by elevated plasma pTau181. Path analysis revealed that neural selectivity at encoding mediated the effects of age, top-down attention, and pTau181 on associative memory. Collectively, these data reveal multiple pathways that contribute to memory differences among older adults -- AD-independent reductions in top-down attention and AD-related pathology alter the precision of cortical representations of events during experience, with consequences for remembering.
Keywords: Alzheimer’s disease; Psychological and Cognitive Neurosciences; Social Sciences; aging; attention; episodic memory; functional MRI; neural selectivity.
Conflict of interest statement
Competing interests: The authors declare no compete of interests.
Figures



References
-
- Kahana M. J., Wagner A. D., Oxford Handbook of Human Memory (Oxford University Press, 2024; https://memory.psych.upenn.edu/Oxford_Handbook_of_Human_Memory).
-
- Cabeza R., Albert M., Belleville S., Craik F. I. M., Duarte A., Grady C. L., Lindenberger U., Nyberg L., Park D. C., Reuter-Lorenz P. A., Rugg M. D., Steffener J., Rajah M. N., Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci 19, 701–710 (2018). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
